Thu, Jan 29, 2026
Volume 7, Issue 1 (3-2018)
2018, 7(1): 43-50 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rastin V, Azizi B, Modabbernia S, Fayazi N, Rahimi M. Evaluation of Periodontal Disease Effect on the Expression of Vascular Endothelial Growth Factor and Nerve Growth Factor in Dental Pulp. Journal title 2018; 7 (1) :43-50
URL: http://3dj.gums.ac.ir/article-1-305-en.html
URL: http://3dj.gums.ac.ir/article-1-305-en.html
1- Assistant Professor, Department of Oral & Maxillofacial Pathology, Faculty of Dentistry, Kurdistan University of Medical Sciences, Sanandaj, Iran.
2- Assistant Professor, Department of Periodontics, Faculty of Dentistry, Kurdistan University of Medical Sciences, Sanandaj, Iran. ,bahareazizi64@gmail.com
3- Assistant Professor, Department of Oral & Maxillofacial Pathology, Faculty of Dentistry, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Oral & Maxillofacial Pathology, Faculty of Dentistry, Kurdistan University of Medical Sciences, Sanandaj, Iran.
2- Assistant Professor, Department of Periodontics, Faculty of Dentistry, Kurdistan University of Medical Sciences, Sanandaj, Iran. ,
3- Assistant Professor, Department of Oral & Maxillofacial Pathology, Faculty of Dentistry, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Oral & Maxillofacial Pathology, Faculty of Dentistry, Kurdistan University of Medical Sciences, Sanandaj, Iran.
Keywords: Immunohistochemistry, Vascular Endothelial Growth Factor (VEGF), Nerve Growth Factor (NGF), Severe periodontitis, Dental Pulp
Full-Text [PDF 1755 kb]
(1135 Downloads)
| Abstract (HTML) (3608 Views)
In this study, the Mean±SD incidence of the VEGF markers in colored cells of group A was 0.345±0.059 and the mean incidence of the VEGF marker in group B cells was 0.354±0.051 cells. Also, the mean incidence of NGF markers in group A cells were 349±0.054 cells and in the cells of the group B was 0.354±0.051. Therefore, there was no significant difference between the ratio of VEGF and NGF between the two groups of patients with severe periodontitis and control (P> 0.05).
There was no significant difference between the color intensity of the patient and the control group in the qualitative study (P<0.05). In Johnson et al. study, the amount of VEGF markers was the lowest in the normal gingiva and in the gingival with a depth to 4 mm was the highest, which indicates a relationship between VEGF and inflammation in periodontal tissues and was not consistent with the results of this study. Considering that all teeth in the case group had severe periodontitis, it may be due to the type of pulp response to stimulation and the duration of periodontitis, which can stimulate a chronic phase of inflammation (including some degrees of atrophy and fibrosis). Thus, in these processes there will not be an increase in the expected expression of VEGF [15].
The study by Unln et al. indicates that diabetic patients with periodontal problems had the highest levels of VEGF, which is associated with microangiopathy and increased angiogenesis in patients with diabetes mellitus [6]. The evaluation of histopathology of tooth tissue in the study of Torabinejad et al. on 25 teeth with periodontal disease, 18 moderate periodontal disease, and 7 severe periodontitis (according to Russell Index), showed no histopathological changes in the pulps of the studied teeth. These results are consistent with the results of our study, in which the marker of vascular endothelial growth in the teeth with severe periodontal disease did not express an increase [16].
Inflamed tissues exhibit excessive expression of NGF [17]. The role of the NGF in the metabolism and tissue repair has been taken into consideration and NGF has been considered as a factor in the organization of periodontal ligaments and alveolar bone during orthodontic dental movements. An increase in the NGF can be explained by its role in inflammation and immune response. In O’Hara et al. study, the immunohistochemical recognition of NGF and its receptors in periodontal ligaments during dental movements was measured.
O’Hara study was performed on 42 mice divided into two groups. In the first group, only dental movements were performed, and in the second group, they had dental movements and anti-NGF injections. These orthodontic movements were performed on each sample on one side of the jaw and compared to the opposite side. This comparison was done on days 0, 3, 7 and 14. The results showed the apparent increase in the incidence of NGF, especially on the seventh day, and this increase was observed in pulp, periodontal ligament and bone, also along with increased secretion by fibroblast cells.
The results of this study were in contrast with our study, which may be due to the type of orthodontic movement, the degree of inflammation, and the differences in the type and number of statistical population. Additionally, the difference in the time intervals for examining samples can also affect the obtained results, because severe chronic periodontal disease is a chronic and long-term process that can produce adaptive responses in the tissues around [8].
In a study conducted by Laurina et al. to investigate the quantitative and qualitative association of NGF and several other growth factors in healthy and diseased periodontal tissues (6 patients with chronic periodontitis and 5 in the control group), they concluded that there was a significant increase in NGF in the periodontal tissue of the patient that may be due to its role in the adaptive process, during periodontitis. These results were also inconsistent with our study results. This inconsistency is the result of difference in the type of studied tissue, pulp in the present study, and periodontal tissue in the Laurina et al. study, as well as the difference in the number of study samples [17].
On the other hand, cement in tooth structure acts as a protective barrier, but if the cement in the CDJ area does not evolve, direct link between the pulp and the periodonum is restored through dentin tubules. Cement may not be present due to developmental defects, disease processes, surgical procedures, or periodontal disease. Exposed dentin tubules in cement areas may act as pathways between the pulp and PDL. Inflammatory changes have been reported near the accessory channels that exposed to periodontitis. In addition, it is hypothesized that the presence of a healthy cementum layer can protect pulp from microbial plaque, which may confirm the results of our study that extremely severe periodontal disease, with a layer of cementum, could not affect the dental pulp [5, 18, 19].
The examination of a cementum layer on the teeth requires more advanced instrumental techniques, such as ultrastructural studies, along with electronic microscope equipment, which because of our limited facilities, this examination was not possible. However, according to our study, the effect of the periodontal disease on the dental pulp could not be observed because of the existence of this protective barrier around the teeth. Czarnecki conducted a study on dental pulp in teeth with varying degrees of periodontal disease. They suggested that periodontal disease did not affect the pulp unless at least the apex had been involved.
This is similar to our study findings in which clinical evaluation indicates no evidence of tooth apex involvement found in the periodontal group [20]. One of the symptoms recorded in the present study is the presence of dentin sensitivity or the root of the teeth sensitivity, that the absence of this symptom in group A and B did not provide the possibility of comparing the two groups and also the possibility of examining the relationship between the presence of sensitivity and the incidence of the NGF. Although the incidence and severity of the VEGF and NGF were not statistically different in the present study, there was little difference in the number and severity of colored cells between these two groups, which may be because of the role of VEGF and NGF in inflamed tissues. However, more studies are needed with a larger statistical sample size with ultrastructure investigations.
5. Conclusion
According to the study results, the number of colored cells and the color intensity of the VEGF and NGF markers in both groups did not show a significant difference, which may confirm that periodontal diseases could not affect the dentin pulp in inflammation and elevation of the vascular endothelial growth factor as well as the growth factor of the nervous system.
Ethical Considerations
Compliance with ethical guidelines
There was no ethical considerations to be considered in this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Full-Text: (1541 Views)
1. Introduction
Researchers have always been interested in the close and reciprocal relationship between the pulps and periodontium tissues. The effects of periodontal disease on human pulp were first reported by Turner and Drew in 1919. They found that bacteria were present in the root canal of the teeth with periodontal lesions but not in the healthy dental pulps [1, 2]. Some researchers also believe that chronic infection in the pulp tissue leads to secondary infection and periodontal degeneration. In contrast, severe periodontal disease can also cause or exacerbate inflammatory changes in the pulp tissue. This relation is physically established through the apical foramen, accessory channels, and dentinal tubules [3].
According to Walton and Granick, the spread of stimulants from the pulp to periodontal tissues is mostly observed around the apex and especially apical foramen and accessory channels [4]. Torabinejad et al. study reports no change in the pulp of the teeth with periodontitis [5].
The primary response of the tooth pulp to damage is similar to other tissues. However, due to the presence of hard dental walls in the pulp chamber, its final outcome can be significantly different when the external stimulation level reaches a critical level, and the mast cell degeneration, decreased blood flow, and cellular damages occur. In the meanwhile, numerous mediators of inflammation are released. These mediators cause vasodilatation, increased vascular flow, and vascular leakage along with edema. Angiogenesis means the formation of new vascular vessels from the vascularity that existed before.
This process results in the division of endothelial cells. Angiogenesis occurs under physiological and pathological conditions and is associated with some degrees of inflammation, which by the creation of small blood vessels leads to the transfer of pro-inflammatory cells, nutrients, and oxygen to inflammatory tissue. Many cytokines and growth factors are involved in the angiogenesis process, but Vascular Endothelial Growth Factor (VEGF) is the key factor in this process [6].
VEGF is essentially a heparin connected to a 45-kDa glycoprotein. The VEGF family consists of 6 members; VEGF, VEGF-E, VEGF-D, VEGF-C, VEGF-B, VEGF-A, among which VEGF-B is the most influential factor in angiogenesis. This factor stimulates the proliferation of endothelial cells, secretion of proteolytic enzymes, as well as chemotaxis and migration. VEGF also affects the endothelial cells. It plays a role in the course of normal growth and tissue regeneration, even in tumors and other pathological conditions, such as delayed allergic reaction and rheumatoid arthritis. Recently, it has been observed that, in the dentin matrix, VEGF expresses during the development of caries, and contributes to the compensatory response of the dentin-pulp complex. These factors are released by monocytes, and the macrophages of the patients with periodontitis in response to bacterial agents.
Regarding to what was discussed, VEGF seems to play a significant role in periodontal diseases [7]. In addition, Neural Growth Factor (NGF) is a polypeptide neurotrophin which is essential for the growth, maintenance, and survival of sympathetic and sensory nerves. This factor is essential for the maintenance of cholinergic nerves in the brain, sympathetic nerves and sensory peripheral tissues, as well as the development and differentiation of these nerves [8].
NGF and its receptors, P75, and (TrkA [Tyrosine Receptor ankylase A]) are also increasing under trauma, transverse nerve cut, dental orthodontic movements, and inflammation. Additionally, mRNA, for NGF, has been identified in human periodontal ligament cells and gingival fibroblasts in the laboratory [8, 9]. The vascular and nervous components are in contact with each other, also the immune cells with vascular endothelium are in contact with each other and have a close proximity to free nerve terminals. This common biochemical association between immune, neurological, and vascular systems indicates the existence of an important functional unit in the pulp [10].
Considering the different views on the effect of periodontal disease on dental pulp, this study aimed to examine the effects of periodontal disease on the expression of VEGF and NGF in dental pulp. This study compared the effects of dental pulp changes through immunohistochemical examination of VEGF and NGF, in the pulp of the teeth with periodontitis as well as healthy teeth pulp.
2. Materials and Methods
This study was an analytical cross-sectional study. The study population comprised all patients referred to periodontics and oral medicine department of the Sanandaj Dental Faculty in 2015-2016. Samples consisted of 30 single-rooted teeth, 15 of which belonged to individuals with severe chronic periodontitis, and 15 others belonged to subjects with a normal periodontal condition. The patients with no history of periodontal surgery were included in the study after completing the forms containing demographic information and clinical and radiographic examination and obtaining their informed consent.
People with the history of taking osteoporotic and steroid anti-inflammatory drugs, taking antibiotics over the past 3 months, having systemic diseases affecting periodontium like diabetes, history of smoking, history of root canal therapy or root canal surgery, history of trauma and root or crown fracture, history of caries or filling or crowning, calcification pulp (based on radiographic evaluation), root resorption, excessive occlusal force (parafunctional habits or Trauma From Occlusions [TFOs]) were excluded from the study.
The patients with hopeless teeth and mobility conditions, advanced and severe periodontal disease (Clinical Attachment Level [CAL] ≥5), the presence of inaccessible areas were extracted atraumatically under local anesthesia, after recording parameters, including bleeding on probing, pus discharge from gingival sulcus, the degree of mobility of the tooth, and the depth of the gingival sulcus at three points around the tooth. In the control group, healthy teeth were extracted during prosthetic and orthodontic treatment. Immediately after tooth extraction, the root end was cut by diamond disk and the tooth was placed in 10% formalin solution.
After encoding, it was transferred to the laboratory. In the laboratory, the teeth were placed in 20% formalic acid solution for decalcification for 21 days. After decalcification, longitudinal sections with a diameter of 40 to 30 μm were performed on the root of the tooth and the slides were prepared in two series. The laboratory method used in the study is immunohistochemistry, which is widely used for diagnostic and research purposes in identifying specific proteins (or other molecules) in cells and tissues. In this method, an antibody is needed against the protein which is going to be detected.
The protein must be purified so that antibodies can be produced against it. To remove additional paraffin from the slides, the samples were cut and placed in an oven for 24 hours at a temperature of 58°C to remove additional paraffins from the slides. Then, the samples were placed on each side on the xylene 100%, for 5 minutes. After removal, the samples were placed in ethanol 100% and then 96% for 5 minutes followed by 3 minutes in 70% ethanol to eliminate residual paraffin residues. Next, they were rinsed on both sides with distilled water for 5 minutes. After this stage, the antigen retrieval samples were put in a plastic container containing buffer citrate; pH=9, at different times; at first 5 minutes at 850 W and then 15 minutes at 450 W in the microwave. After 15 minutes, the specimens were washed for 15 minutes. After drying the slides, peroxidase solution 3% was poured onto each sample and kept it in a dark and humid chamber for 20 minutes. Subsequently, the samples were washed in PBS for 5 minutes.
Primary monoclonal antibody
VEGF (Mouse monoclonal antibody; code M7273, Dako, USA) and NGF (mouse monoclonal antibody, code M7273, Dako, USA) were sprayed onto each slide series individually, and washed at room temperature in a dark and humid chamber in 2 PBS buffers dishes for 5 minutes each time. All of the stained slides were examined by VEGF and NGF markers with a Motic type 102 Educational Microscope (MIGS) (made in China) under 40x magnification, by oral and maxillofacial pathologists. The number of cells stained with VEGF and NGF markers was determined by counting all areas of a slide, and then the number of colored cells was calculated as percentages for each sample and index labels was performed. In order to avoid the counting errors, each section was counted twice.
Samples were qualitatively graded into 3 groups [11]. Grade 0: Lack of coloring; Grade 1: Low to moderate coloring; the cytoplasm and the nucleolus were not stained completely; Grade 2: High cytoplasmic and nucleolus staining; The cell is completely stained in brown-black color. The statistical tests used in this study were the Independent t test and Fisher Exact test. The significance level in all tests was less than 0.05.
3. Results
Of 30 examined teeth, 15 were from individuals with severe chronic periodontitis (group A), and 15 from individuals without any periodontal disease (group B). The mean age of the participants in the group A was 42.54 years and in the group B was 22.14 years. Table 1 shows the clinical information of the patients. Bleeding on Probing (BOP) was seen in all teeth in the disease group, and pus secretion in 5 of them. The maximum gingival recession (CEJ [Cemento-Enamel Junction] to gingival margin) was 7 and at least 3 mm. The bone loss, performed by using the periapical graph (from CEJ to alveolar crest), was reported to be from 3 mm to maximum 12 mm. Gingival sulcular depth measurements were also carried out. They are all presented in Table 2.
Researchers have always been interested in the close and reciprocal relationship between the pulps and periodontium tissues. The effects of periodontal disease on human pulp were first reported by Turner and Drew in 1919. They found that bacteria were present in the root canal of the teeth with periodontal lesions but not in the healthy dental pulps [1, 2]. Some researchers also believe that chronic infection in the pulp tissue leads to secondary infection and periodontal degeneration. In contrast, severe periodontal disease can also cause or exacerbate inflammatory changes in the pulp tissue. This relation is physically established through the apical foramen, accessory channels, and dentinal tubules [3].
According to Walton and Granick, the spread of stimulants from the pulp to periodontal tissues is mostly observed around the apex and especially apical foramen and accessory channels [4]. Torabinejad et al. study reports no change in the pulp of the teeth with periodontitis [5].
The primary response of the tooth pulp to damage is similar to other tissues. However, due to the presence of hard dental walls in the pulp chamber, its final outcome can be significantly different when the external stimulation level reaches a critical level, and the mast cell degeneration, decreased blood flow, and cellular damages occur. In the meanwhile, numerous mediators of inflammation are released. These mediators cause vasodilatation, increased vascular flow, and vascular leakage along with edema. Angiogenesis means the formation of new vascular vessels from the vascularity that existed before.
This process results in the division of endothelial cells. Angiogenesis occurs under physiological and pathological conditions and is associated with some degrees of inflammation, which by the creation of small blood vessels leads to the transfer of pro-inflammatory cells, nutrients, and oxygen to inflammatory tissue. Many cytokines and growth factors are involved in the angiogenesis process, but Vascular Endothelial Growth Factor (VEGF) is the key factor in this process [6].
VEGF is essentially a heparin connected to a 45-kDa glycoprotein. The VEGF family consists of 6 members; VEGF, VEGF-E, VEGF-D, VEGF-C, VEGF-B, VEGF-A, among which VEGF-B is the most influential factor in angiogenesis. This factor stimulates the proliferation of endothelial cells, secretion of proteolytic enzymes, as well as chemotaxis and migration. VEGF also affects the endothelial cells. It plays a role in the course of normal growth and tissue regeneration, even in tumors and other pathological conditions, such as delayed allergic reaction and rheumatoid arthritis. Recently, it has been observed that, in the dentin matrix, VEGF expresses during the development of caries, and contributes to the compensatory response of the dentin-pulp complex. These factors are released by monocytes, and the macrophages of the patients with periodontitis in response to bacterial agents.
Regarding to what was discussed, VEGF seems to play a significant role in periodontal diseases [7]. In addition, Neural Growth Factor (NGF) is a polypeptide neurotrophin which is essential for the growth, maintenance, and survival of sympathetic and sensory nerves. This factor is essential for the maintenance of cholinergic nerves in the brain, sympathetic nerves and sensory peripheral tissues, as well as the development and differentiation of these nerves [8].
NGF and its receptors, P75, and (TrkA [Tyrosine Receptor ankylase A]) are also increasing under trauma, transverse nerve cut, dental orthodontic movements, and inflammation. Additionally, mRNA, for NGF, has been identified in human periodontal ligament cells and gingival fibroblasts in the laboratory [8, 9]. The vascular and nervous components are in contact with each other, also the immune cells with vascular endothelium are in contact with each other and have a close proximity to free nerve terminals. This common biochemical association between immune, neurological, and vascular systems indicates the existence of an important functional unit in the pulp [10].
Considering the different views on the effect of periodontal disease on dental pulp, this study aimed to examine the effects of periodontal disease on the expression of VEGF and NGF in dental pulp. This study compared the effects of dental pulp changes through immunohistochemical examination of VEGF and NGF, in the pulp of the teeth with periodontitis as well as healthy teeth pulp.
2. Materials and Methods
This study was an analytical cross-sectional study. The study population comprised all patients referred to periodontics and oral medicine department of the Sanandaj Dental Faculty in 2015-2016. Samples consisted of 30 single-rooted teeth, 15 of which belonged to individuals with severe chronic periodontitis, and 15 others belonged to subjects with a normal periodontal condition. The patients with no history of periodontal surgery were included in the study after completing the forms containing demographic information and clinical and radiographic examination and obtaining their informed consent.
People with the history of taking osteoporotic and steroid anti-inflammatory drugs, taking antibiotics over the past 3 months, having systemic diseases affecting periodontium like diabetes, history of smoking, history of root canal therapy or root canal surgery, history of trauma and root or crown fracture, history of caries or filling or crowning, calcification pulp (based on radiographic evaluation), root resorption, excessive occlusal force (parafunctional habits or Trauma From Occlusions [TFOs]) were excluded from the study.
The patients with hopeless teeth and mobility conditions, advanced and severe periodontal disease (Clinical Attachment Level [CAL] ≥5), the presence of inaccessible areas were extracted atraumatically under local anesthesia, after recording parameters, including bleeding on probing, pus discharge from gingival sulcus, the degree of mobility of the tooth, and the depth of the gingival sulcus at three points around the tooth. In the control group, healthy teeth were extracted during prosthetic and orthodontic treatment. Immediately after tooth extraction, the root end was cut by diamond disk and the tooth was placed in 10% formalin solution.
After encoding, it was transferred to the laboratory. In the laboratory, the teeth were placed in 20% formalic acid solution for decalcification for 21 days. After decalcification, longitudinal sections with a diameter of 40 to 30 μm were performed on the root of the tooth and the slides were prepared in two series. The laboratory method used in the study is immunohistochemistry, which is widely used for diagnostic and research purposes in identifying specific proteins (or other molecules) in cells and tissues. In this method, an antibody is needed against the protein which is going to be detected.
The protein must be purified so that antibodies can be produced against it. To remove additional paraffin from the slides, the samples were cut and placed in an oven for 24 hours at a temperature of 58°C to remove additional paraffins from the slides. Then, the samples were placed on each side on the xylene 100%, for 5 minutes. After removal, the samples were placed in ethanol 100% and then 96% for 5 minutes followed by 3 minutes in 70% ethanol to eliminate residual paraffin residues. Next, they were rinsed on both sides with distilled water for 5 minutes. After this stage, the antigen retrieval samples were put in a plastic container containing buffer citrate; pH=9, at different times; at first 5 minutes at 850 W and then 15 minutes at 450 W in the microwave. After 15 minutes, the specimens were washed for 15 minutes. After drying the slides, peroxidase solution 3% was poured onto each sample and kept it in a dark and humid chamber for 20 minutes. Subsequently, the samples were washed in PBS for 5 minutes.
Primary monoclonal antibody
VEGF (Mouse monoclonal antibody; code M7273, Dako, USA) and NGF (mouse monoclonal antibody, code M7273, Dako, USA) were sprayed onto each slide series individually, and washed at room temperature in a dark and humid chamber in 2 PBS buffers dishes for 5 minutes each time. All of the stained slides were examined by VEGF and NGF markers with a Motic type 102 Educational Microscope (MIGS) (made in China) under 40x magnification, by oral and maxillofacial pathologists. The number of cells stained with VEGF and NGF markers was determined by counting all areas of a slide, and then the number of colored cells was calculated as percentages for each sample and index labels was performed. In order to avoid the counting errors, each section was counted twice.
Samples were qualitatively graded into 3 groups [11]. Grade 0: Lack of coloring; Grade 1: Low to moderate coloring; the cytoplasm and the nucleolus were not stained completely; Grade 2: High cytoplasmic and nucleolus staining; The cell is completely stained in brown-black color. The statistical tests used in this study were the Independent t test and Fisher Exact test. The significance level in all tests was less than 0.05.
3. Results
Of 30 examined teeth, 15 were from individuals with severe chronic periodontitis (group A), and 15 from individuals without any periodontal disease (group B). The mean age of the participants in the group A was 42.54 years and in the group B was 22.14 years. Table 1 shows the clinical information of the patients. Bleeding on Probing (BOP) was seen in all teeth in the disease group, and pus secretion in 5 of them. The maximum gingival recession (CEJ [Cemento-Enamel Junction] to gingival margin) was 7 and at least 3 mm. The bone loss, performed by using the periapical graph (from CEJ to alveolar crest), was reported to be from 3 mm to maximum 12 mm. Gingival sulcular depth measurements were also carried out. They are all presented in Table 2.
In group A with severe chronic periodontitis, the number of patients with different mobility teeth degree were as follows: first grade mobility teeth: 3; second grade mobility teeth: 5; third grade mobility teeth:7.
Immunohistochemical findings
The study of samples with VEGF and NGF markers
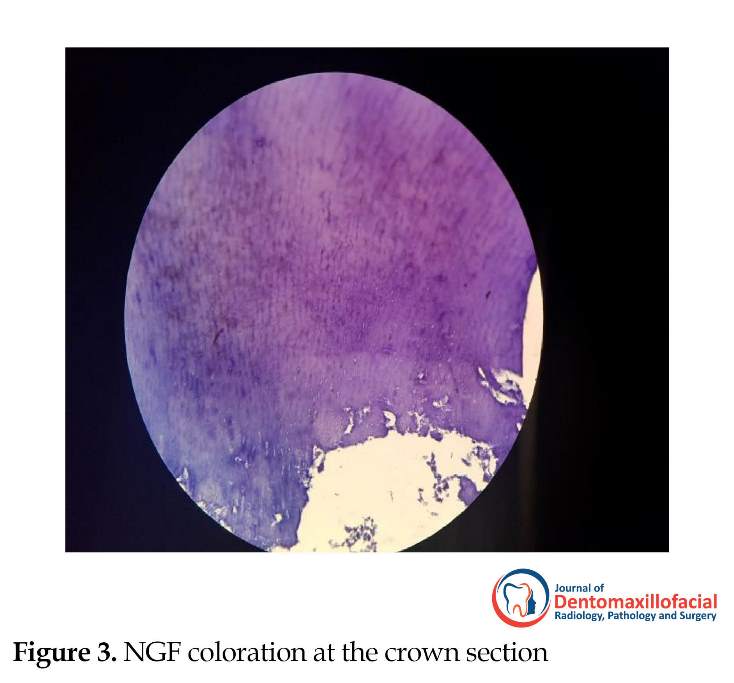
The cytoplasmic and nuclear staining of the cells in the samples were considered as positive immunoreactivity with the markers of VEGF and NGF (Figures 1, 2 and 3).
The count of the colored cells with the VEGF and NGF markers in each group was performed in accordance with the methodology of the study that was previously explained, and the following results were obtained. The Mean±SD occurrence of a VEGF marker in the pulp cells of the teeth with periodontitis was 0.345±0.059 cells and in the same group for the NGF marker were 0.349±0.05 cells. The Mean±SD incidence of VEGF markers in healthy periodontal status was 0.345±0.051 and for NGF markers were 0.052±0.0345 (Table 3).
To compare the ratio of colored cells between the two groups, the Independent t test was used. There was no significant difference between the ratio of VEGF and NGF in the severity of periodontitis and control group in terms of incidence (P<0.05).
Immunohistochemical findings
The study of samples with VEGF and NGF markers
The cytoplasmic and nuclear staining of the cells in the samples were considered as positive immunoreactivity with the markers of VEGF and NGF (Figures 1, 2 and 3).
The count of the colored cells with the VEGF and NGF markers in each group was performed in accordance with the methodology of the study that was previously explained, and the following results were obtained. The Mean±SD occurrence of a VEGF marker in the pulp cells of the teeth with periodontitis was 0.345±0.059 cells and in the same group for the NGF marker were 0.349±0.05 cells. The Mean±SD incidence of VEGF markers in healthy periodontal status was 0.345±0.051 and for NGF markers were 0.052±0.0345 (Table 3).
To compare the ratio of colored cells between the two groups, the Independent t test was used. There was no significant difference between the ratio of VEGF and NGF in the severity of periodontitis and control group in terms of incidence (P<0.05).
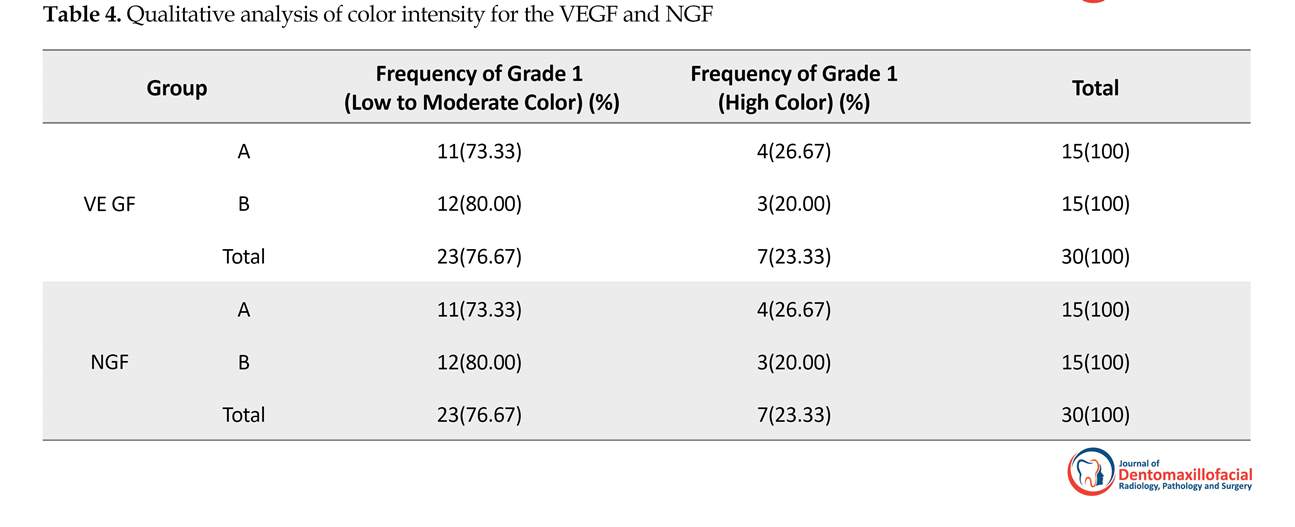
In this study, in addition to quantitative study of VEGF and NGF, a qualitative method was used. Fisher analysis was used in qualitative analysis, which was done for the color intensity of the samples (P<0.05). There was no statistically significant difference between two groups in terms of color intensity, which is presented in Table 4.
4. Discussion
The relationship between endodontic lesions and periodontal diseases is very important, because these diseases can be challenging for clinicians in order to choose the treatment process and the determination of prognosis of periodontal-endodontic diseases. Moreover, understanding the relationship between endodontic and periodontal diseases empowers clinicians to select the best treatment plan. The direct relationship between dental pulp and periodontal disease may occur through three main ways: Dental tubules; Lateral channels; and Apical foramen [5].
Seltzer believed that lateral channels that are open into the oral cavity could transfer toxic products to the pulp, causing degeneration, atrophy, inflammation, and burnout in the pulp. On the other hand, Stanly believed that if the dentin thickness between the pulp and the stimulated area was 2 mm, there would be little chance that the tooth pulp would be degraded. In addition, some believe that by using the scaling and root planning the pulp will not be compromised, even if there are clinical signs of pulpitis [12, 13]. On the other hand, Abed and Kamali et al. who studied the effects of advanced periodontal disease on pulp status, concluded that periodontal disease significantly affected pulp tissue [14].
4. Discussion
The relationship between endodontic lesions and periodontal diseases is very important, because these diseases can be challenging for clinicians in order to choose the treatment process and the determination of prognosis of periodontal-endodontic diseases. Moreover, understanding the relationship between endodontic and periodontal diseases empowers clinicians to select the best treatment plan. The direct relationship between dental pulp and periodontal disease may occur through three main ways: Dental tubules; Lateral channels; and Apical foramen [5].
Seltzer believed that lateral channels that are open into the oral cavity could transfer toxic products to the pulp, causing degeneration, atrophy, inflammation, and burnout in the pulp. On the other hand, Stanly believed that if the dentin thickness between the pulp and the stimulated area was 2 mm, there would be little chance that the tooth pulp would be degraded. In addition, some believe that by using the scaling and root planning the pulp will not be compromised, even if there are clinical signs of pulpitis [12, 13]. On the other hand, Abed and Kamali et al. who studied the effects of advanced periodontal disease on pulp status, concluded that periodontal disease significantly affected pulp tissue [14].
In this study, the Mean±SD incidence of the VEGF markers in colored cells of group A was 0.345±0.059 and the mean incidence of the VEGF marker in group B cells was 0.354±0.051 cells. Also, the mean incidence of NGF markers in group A cells were 349±0.054 cells and in the cells of the group B was 0.354±0.051. Therefore, there was no significant difference between the ratio of VEGF and NGF between the two groups of patients with severe periodontitis and control (P> 0.05).
There was no significant difference between the color intensity of the patient and the control group in the qualitative study (P<0.05). In Johnson et al. study, the amount of VEGF markers was the lowest in the normal gingiva and in the gingival with a depth to 4 mm was the highest, which indicates a relationship between VEGF and inflammation in periodontal tissues and was not consistent with the results of this study. Considering that all teeth in the case group had severe periodontitis, it may be due to the type of pulp response to stimulation and the duration of periodontitis, which can stimulate a chronic phase of inflammation (including some degrees of atrophy and fibrosis). Thus, in these processes there will not be an increase in the expected expression of VEGF [15].
The study by Unln et al. indicates that diabetic patients with periodontal problems had the highest levels of VEGF, which is associated with microangiopathy and increased angiogenesis in patients with diabetes mellitus [6]. The evaluation of histopathology of tooth tissue in the study of Torabinejad et al. on 25 teeth with periodontal disease, 18 moderate periodontal disease, and 7 severe periodontitis (according to Russell Index), showed no histopathological changes in the pulps of the studied teeth. These results are consistent with the results of our study, in which the marker of vascular endothelial growth in the teeth with severe periodontal disease did not express an increase [16].
Inflamed tissues exhibit excessive expression of NGF [17]. The role of the NGF in the metabolism and tissue repair has been taken into consideration and NGF has been considered as a factor in the organization of periodontal ligaments and alveolar bone during orthodontic dental movements. An increase in the NGF can be explained by its role in inflammation and immune response. In O’Hara et al. study, the immunohistochemical recognition of NGF and its receptors in periodontal ligaments during dental movements was measured.
O’Hara study was performed on 42 mice divided into two groups. In the first group, only dental movements were performed, and in the second group, they had dental movements and anti-NGF injections. These orthodontic movements were performed on each sample on one side of the jaw and compared to the opposite side. This comparison was done on days 0, 3, 7 and 14. The results showed the apparent increase in the incidence of NGF, especially on the seventh day, and this increase was observed in pulp, periodontal ligament and bone, also along with increased secretion by fibroblast cells.
The results of this study were in contrast with our study, which may be due to the type of orthodontic movement, the degree of inflammation, and the differences in the type and number of statistical population. Additionally, the difference in the time intervals for examining samples can also affect the obtained results, because severe chronic periodontal disease is a chronic and long-term process that can produce adaptive responses in the tissues around [8].
In a study conducted by Laurina et al. to investigate the quantitative and qualitative association of NGF and several other growth factors in healthy and diseased periodontal tissues (6 patients with chronic periodontitis and 5 in the control group), they concluded that there was a significant increase in NGF in the periodontal tissue of the patient that may be due to its role in the adaptive process, during periodontitis. These results were also inconsistent with our study results. This inconsistency is the result of difference in the type of studied tissue, pulp in the present study, and periodontal tissue in the Laurina et al. study, as well as the difference in the number of study samples [17].
On the other hand, cement in tooth structure acts as a protective barrier, but if the cement in the CDJ area does not evolve, direct link between the pulp and the periodonum is restored through dentin tubules. Cement may not be present due to developmental defects, disease processes, surgical procedures, or periodontal disease. Exposed dentin tubules in cement areas may act as pathways between the pulp and PDL. Inflammatory changes have been reported near the accessory channels that exposed to periodontitis. In addition, it is hypothesized that the presence of a healthy cementum layer can protect pulp from microbial plaque, which may confirm the results of our study that extremely severe periodontal disease, with a layer of cementum, could not affect the dental pulp [5, 18, 19].
The examination of a cementum layer on the teeth requires more advanced instrumental techniques, such as ultrastructural studies, along with electronic microscope equipment, which because of our limited facilities, this examination was not possible. However, according to our study, the effect of the periodontal disease on the dental pulp could not be observed because of the existence of this protective barrier around the teeth. Czarnecki conducted a study on dental pulp in teeth with varying degrees of periodontal disease. They suggested that periodontal disease did not affect the pulp unless at least the apex had been involved.
This is similar to our study findings in which clinical evaluation indicates no evidence of tooth apex involvement found in the periodontal group [20]. One of the symptoms recorded in the present study is the presence of dentin sensitivity or the root of the teeth sensitivity, that the absence of this symptom in group A and B did not provide the possibility of comparing the two groups and also the possibility of examining the relationship between the presence of sensitivity and the incidence of the NGF. Although the incidence and severity of the VEGF and NGF were not statistically different in the present study, there was little difference in the number and severity of colored cells between these two groups, which may be because of the role of VEGF and NGF in inflamed tissues. However, more studies are needed with a larger statistical sample size with ultrastructure investigations.
5. Conclusion
According to the study results, the number of colored cells and the color intensity of the VEGF and NGF markers in both groups did not show a significant difference, which may confirm that periodontal diseases could not affect the dentin pulp in inflammation and elevation of the vascular endothelial growth factor as well as the growth factor of the nervous system.
Ethical Considerations
Compliance with ethical guidelines
There was no ethical considerations to be considered in this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
- Seltzer S, Bender IB. Biologic Consideration of Pulp. Texas: Ishizaku Euro America; 1990.
- Sadeghi R, Nazari Moghaddam K, Jooyandeh J. [Clinical evaluation of endodotic therapy on periodontal tissue healing in chronic advanced periodontitis (Persian)]. Journal of Dental Medicine. 2004; 17(3):83-91.
- Cohen S, Hargreaves KM. Pathways of the pulp. Maryland Heights: Mosby; 2006.
- Walton RE, Garnick JJ. The histology of periapical inflammatory lesions in permanent molars in monkeys. Journal of Endodontics. 1986; 12(2):49-53. [DOI:10.1016/S0099-2399(86)80127-3]
- Fouad A, Torabinejad M, Walton RE. Endodontics-E-Book: Principles and practice. Amsterdam: Elsevier Health Sciences; 2008.
- Unlu F,Guneri PG,Hekimgil M,Yesilbek B,Boyacioglu H. Expression of vascular endothelial growth factor in human periodontal tissue : Comparison of healthy and diabetic patient. Journal of Periodontology. 2003 ; 74(2):181-7. [DOI:10.1902/jop.2003.74.2.181] [PMID]
- Grando ML, Westphalen BL, de Figueiredo J, Nör J, de Araujo F, Fossati A. Vascular endothelial growth factor and its relationship with the dental pulp. Journal of Endodontics. 2007; 33(5):524-30. [DOI:10.1016/j.joen.2007.01.003] [PMID]
- O’Hara AH, Sampson WJ, Dreyer CW, Pierce AM, Ferguson IA. Immunohistochemical detection of nerve growth factor and its receptors in the rat periodontal ligament during tooth movement. Archives of Oral Biology. 2009; 54(9):871-8. [DOI:10.1016/j.archoralbio.2009.06.003] [PMID]
- Chesa P, Rettig W, Thomson T, Old L, Melamed M. Immunohistochemical analysis of nerve growth factor receptor expression in normal and malignant human tissues. The Journal of Histochemistry and Cytochemistry. 1988; 36(4):383-9. [DOI:10.1177/36.4.2831267] [PMID]
- Nanci A, Ten Cate AR. Ten cate’s oral histology-pageburst on vitalsource: Development, structure, and function. Amsterdam: Elsevier Health Sciences; 2008.
- Rubach WC, Mitchell DF. Periodontal disease, accessory canals and pulp pathosis. Journal of Periodontology. 1965; 36(1):34-8. [DOI:10.1902/jop.1965.36.1.34]
- Seltzer S, Bender IB. The dental pulp: Biologic considerations in dental procedures. Philadelphia: Lippincott Williams & Wilkins; 1975.
- Seyed Rezaei M, Khosravi M, Bijani A, Babak M. [The Effect of Pathology of Advanced Periodontal Disease on Dental Pulps (Persian)]. Journal of Dentistry. 2012; 12(3):233-8.
- Kamali A, Disfani R, Mirzaei M. [Histopathologic study of the effects of advanced periodontal diseases on pulp (Persian)]. Journal of Islamic Dental Association of Iran. 2003; 14(3):30-6.
- Johnson R, Serio F, Dai X. Vascular endothelial growth factors and progression of periodontal diseases. Journal of Periodontology. 1999; 70(8):848-52. [DOI:10.1902/jop.1999.70.8.848] [PMID]
- Torabinejad M, Kiger R. A histologic evaluation of dental pulp tissue of a patient with periodontal disease. Oral Surgery, Oral Medicine, and Oral Pathology. 1985; 59(2):198-200. [DOI:10.1016/0030-4220(85)90018-0]
- Laurina Z, Pilmane M, Care R. Growth factors/cytokines/defensins and apoptosis in periodontal pathologies. Stomatologija. 2009; 11(2):48-54. [PMID]
- Harrington GW, Steiner DR, Ammons Jr WF. The periodontal–endodontic controversy. Periodontology 2000. 2002; 30(1):123-30. [PMID]
- Rotstein I. Interaction between endodontics and periodontics. Periodontology 2000. 2017; 74(1):11-39. [DOI:10.1111/prd.12188] [PMID]
- Czarnecki R, Schilder H. A histological evaluation of the human pulp in teeth with varying degrees of periodontal disease. Journal of ُndodontics. 1979; 5(8):242-53. [DOI:10.1016/S0099-2399(79)80018-7]
Type of Study: Original article |
Subject:
Pathology
Received: 2017/10/14 | Accepted: 2018/01/27 | Published: 2018/03/1
Received: 2017/10/14 | Accepted: 2018/01/27 | Published: 2018/03/1
References
1. Seltzer S, Bender IB. Biologic Consideration of Pulp. Texas: Ishizaku Euro America; 1990. [PMCID]
2. Sadeghi R, Nazari Moghaddam K, Jooyandeh J. [Clinical evaluation of endodotic therapy on periodontal tissue healing in chronic advanced periodontitis (Persian)]. Journal of Dental Medicine. 2004; 17(3):83-91.
3. Cohen S, Hargreaves KM. Pathways of the pulp. Maryland Heights: Mosby; 2006.
4. Walton RE, Garnick JJ. The histology of periapical inflammatory lesions in permanent molars in monkeys. Journal of Endodontics. 1986; 12(2):49-53. [DOI:10.1016/S0099-2399(86)80127-3] [DOI:10.1016/S0099-2399(86)80127-3]
5. Fouad A, Torabinejad M, Walton RE. Endodontics-E-Book: Principles and practice. Amsterdam: Elsevier Health Sciences; 2008.
6. Unlu F,Guneri PG,Hekimgil M,Yesilbek B,Boyacioglu H. Expression of vascular endothelial growth factor in human periodontal tissue : Comparison of healthy and diabetic patient. Journal of Periodontology. 2003 ; 74(2):181-7. [DOI:10.1902/jop.2003.74.2.181] [PMID] [DOI:10.1902/jop.2003.74.2.181]
7. Grando ML, Westphalen BL, de Figueiredo J, Nör J, de Araujo F, Fossati A. Vascular endothelial growth factor and its relationship with the dental pulp. Journal of Endodontics. 2007; 33(5):524-30. [DOI:10.1016/j.joen.2007.01.003] [PMID] [DOI:10.1016/j.joen.2007.01.003]
8. O'Hara AH, Sampson WJ, Dreyer CW, Pierce AM, Ferguson IA. Immunohistochemical detection of nerve growth factor and its receptors in the rat periodontal ligament during tooth movement. Archives of Oral Biology. 2009; 54(9):871-8. [DOI:10.1016/j.archoralbio.2009.06.003] [PMID] [DOI:10.1016/j.archoralbio.2009.06.003]
9. Chesa P, Rettig W, Thomson T, Old L, Melamed M. Immunohistochemical analysis of nerve growth factor receptor expression in normal and malignant human tissues. The Journal of Histochemistry and Cytochemistry. 1988; 36(4):383-9. [DOI:10.1177/36.4.2831267] [PMID] [DOI:10.1177/36.4.2831267]
10. Nanci A, Ten Cate AR. Ten cate's oral histology-pageburst on vitalsource: Development, structure, and function. Amsterdam: Elsevier Health Sciences; 2008. [PMCID]
11. Rubach WC, Mitchell DF. Periodontal disease, accessory canals and pulp pathosis. Journal of Periodontology. 1965; 36(1):34-8. [DOI:10.1902/jop.1965.36.1.34] [DOI:10.1902/jop.1965.36.1.34]
12. Seltzer S, Bender IB. The dental pulp: Biologic considerations in dental procedures. Philadelphia: Lippincott Williams & Wilkins; 1975.
13. Seyed Rezaei M, Khosravi M, Bijani A, Babak M. [The Effect of Pathology of Advanced Periodontal Disease on Dental Pulps (Persian)]. Journal of Dentistry. 2012; 12(3):233-8.
14. Kamali A, Disfani R, Mirzaei M. [Histopathologic study of the effects of advanced periodontal diseases on pulp (Persian)]. Journal of Islamic Dental Association of Iran. 2003; 14(3):30-6.
15. Johnson R, Serio F, Dai X. Vascular endothelial growth factors and progression of periodontal diseases. Journal of Periodontology. 1999; 70(8):848-52. [DOI:10.1902/jop.1999.70.8.848] [PMID] [DOI:10.1902/jop.1999.70.8.848]
16. Torabinejad M, Kiger R. A histologic evaluation of dental pulp tissue of a patient with periodontal disease. Oral Surgery, Oral Medicine, and Oral Pathology. 1985; 59(2):198-200. [DOI:10.1016/0030-4220(85)90018-0] [DOI:10.1016/0030-4220(85)90018-0]
17. Laurina Z, Pilmane M, Care R. Growth factors/cytokines/defensins and apoptosis in periodontal pathologies. Stomatologija. 2009; 11(2):48-54. [PMID]
18. Harrington GW, Steiner DR, Ammons Jr WF. The periodontal–endodontic controversy. Periodontology 2000. 2002; 30(1):123-30. [PMID] [DOI:10.1034/j.1600-0757.2002.03012.x]
19. Rotstein I. Interaction between endodontics and periodontics. Periodontology 2000. 2017; 74(1):11-39. [DOI:10.1111/prd.12188] [PMID] [DOI:10.1111/prd.12188]
20. Czarnecki R, Schilder H. A histological evaluation of the human pulp in teeth with varying degrees of periodontal disease. Journal of ُndodontics. 1979; 5(8):242-53. [DOI:10.1016/S0099-2399(79)80018-7] [DOI:10.1016/S0099-2399(79)80018-7]
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |