Fri, Apr 26, 2024
Volume 7, Issue 1 (3-2018)
2018, 7(1): 1-6 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Jalalian E, Shalchi M, Hajian-Tilaki A, Aghajani Nargesi R. Adhesion of Streptococcus Mutans to Zirconia, Enamel, IPS Empress II, Noble Alloy and Base-metal: An In-Vitro Comparative Study. Journal title 2018; 7 (1) :1-6
URL: http://3dj.gums.ac.ir/article-1-302-en.html
URL: http://3dj.gums.ac.ir/article-1-302-en.html
1- Associate Professor, Department of Prosthodontics, Faculty of Dentistry, Tehran Medical Branch, Islamic Azad University, Tehran, Iran.
2- Assistant Professor, Department of Orthodontics, Faculty of Dentistry, Guilan University of Medical Sciences, Rasht, Iran.
3- Postgraduate Student of Orthodontics, Department of Orthodontics, Faculty of Dentistry, Guilan University of Medical Sciences, Rasht, Iran.
4- Assistant Professor, Department of ProsthodonticAssistant Professor, Department of Prosthodontics, Faculty of Dentistry, Guilan University of Medical Sciences, Rasht, Iran.s, Faculty of Dentistry, Guilan University of Medical Sciences, Rasht, Iran. , arefehhajian69@gmail.com
2- Assistant Professor, Department of Orthodontics, Faculty of Dentistry, Guilan University of Medical Sciences, Rasht, Iran.
3- Postgraduate Student of Orthodontics, Department of Orthodontics, Faculty of Dentistry, Guilan University of Medical Sciences, Rasht, Iran.
4- Assistant Professor, Department of ProsthodonticAssistant Professor, Department of Prosthodontics, Faculty of Dentistry, Guilan University of Medical Sciences, Rasht, Iran.s, Faculty of Dentistry, Guilan University of Medical Sciences, Rasht, Iran. , arefehhajian69@gmail.com
Full-Text [PDF 858 kb]
(824 Downloads)
| Abstract (HTML) (2995 Views)
The hydrophilic properties of the surfaces is effective on the initial nonspecific bacterial adhesion and specific agents on the cellular wall of the bacteria have greater influence on the adhesion [18]. S. mutans adheres to pellicle on the substrate and other bacteria by formation of extracellular glucan from sucrose and adhesion to this polysaccharide by glucan binding proteins [19]. S. mutans can adhere to the saliva mucin by its surface enolase protein [20].
According to this investigation, the adhesion of S. mutans had significant differences among the study groups. The least adhesion was observed with zirconia, tooth enamel, IPS Empress, noble alloy, and the highest level of adhesion was seen to base-metal alloys. In the present study, the differences in the study groups can be directly correlated to the substrate composition that can influence the initial bacterial adhesion and indirectly by differences in the pellicle layer formed on the substrate (specially based on its mucin). In the research studies, different methods like culture and colony numeration under light microscope, observation of bacteria adherence to surface by SEM and measuring the light generated by the bacteria through spectrofluorometer were used [10, 21-23]. In this investigation for evaluating the amount of S. mutans adhesion, culture and colony numeration under light microscope and SEM method were used and the SEM results confirmed the culture results.
The comparison of the studies was difficult because of the different techniques used for evaluating the bacteria adhesion, not using enamel as the control group in some studies and the different surface roughness in studied materials. The use of enamel as the control group results in better comparison between different materials that are not compared with each other in a single investigation. Therefore in conducting this study, enamel was used as the control group [10].
For eliminating the effect of surface roughness on the degree of bacteria adhesion, the disks first went under polishing and then the surface roughness of four study groups and enamel was evaluated by profilometer and the results indicated no statistically significant difference in the paired comparison of the groups. In the current study, the adhesion of S. mutans to the samples made of zirconia ceramics was lower than IPS-Empress which was also statistically significant.
In Kantorski et al. investigation on two types of ceramics; feldespatics and lucite/feldespatics in comparison to enamel, the adhesion of S. mutans to specimens formed by enamel was more than porcelain [23]. In this study the surface roughness of enamel was more than ceramics. Based on the results of our study, there is no significant difference in surface roughness of the studied materials and enamel and adhesion to enamel was higher than zirconia but less than IPS-Empress. In the current study, there was no significant difference between zirconia and IPS-Empress surface roughness but bacterial adhesion to zirconia was lower. In Bremers investigation which was similar to our study with respect to surface roughness, the results were in agreement with our study [24].
According to this study, the adhesion of S. mutans to enamel was less than noble (X33) and base -metal (super one) which is similar to Sardin et al. results. In Sardin et al. investigation, by polishing the surfaces and creating similar surface roughness in the range of 0.1 micron, the influence of surface roughness on bacteria adhesion was eliminated and in comparison with the four alloys, noble and a base-metal alloy (Ni-Cr), the lowest adhesion was observed in enamel [25].
In comparison between noble and base-metal alloys in the current study, the adhesion of S. mutans to noble alloy was lower which is in contrast with Grivet et al. findings. In Grivet et al. study on four precious alloys and base-metal, although the surface roughness of five study groups ranged from 0.02 to 0.06 µm and the influence of surface roughness values on the bacterial adhesion was not statistically significant, the amount of S. mutans adherence was higher in precious group than in base-metal. This difference may be because of the surface free energy of the tested materials, selection of materials from different companies and differences in the methods of investigation [26].
5. Conclusion
Different restorative materials have different bacteria adhesion. Zirconia had the lowest adhesion among the tested materials and the highest adhesion was seen in the base-metal. Based on the findings of the current study, the use of restorative ceramics, including zirconia is a better choice in patients with poor oral hygiene and those who are susceptible to periodontal disease.
Limitation of our study was related to the evaluation of one type of oral bacteria. More studies are recommended on other bacteria and using dynamic procedures like flow chamber to simulate oral environment.
Ethical Considerations
Compliance with ethical guidelines
There was no ethical considerations to be considered in this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Full-Text: (985 Views)
1. Introduction
Microbial adhesion is the initial stage of colonization and biofilm formation in the mouth environment [1, 2]. After biofilm formation, dental plaque forms on the tooth surfaces and intraoral restorations. Dental plaque is the common reason of caries and oral cavity disease such as gingivitis, periodontitis and peri-implantitis [3]. The contributing factors for bacterial adhesion to restorative surfaces consist of surface substance, electrical charge of microbial particles and their antibacterial effect, surface roughness and its hydrophilic and hydrophobic properties, and the surface proteins of the bacterial membrane [3].
Studies have already been conducted on microbial adhesion to enamel, composite, amalgam, ceramic and metal surfaces [4-8]. Although comprehensive investigations about bacterial adhesion to restorative surfaces have been performed, the current studies reveal a difference in surface properties and bacterial adhesion to restorative surfaces [9-12]. Among the bacteria that are effective on adhesion and formation of the dental plaque, Streptococcus Mutans (S. mutans) is the pioneer and has a critical role in caries and periodontal disease. Therefore, evaluation of S. mutans and its colonization on the restorative materials plays a special role on the success of restorations [4, 10, 13].
Mechanical properties, chemical stability and esthetics are the reasons for the selection of the restorative materials [3, 14]. The use of all ceramic restorations, especially zirconia has increased in the past few years because of their excellent esthetic, high strength and biocompatibility [3, 10]. Up to now, a large number of in vitro and in vivo investigations on restorative materials with respect to their mechanical properties, chemical stability and toxicity have been conducted; however, most studies overlooked the topic of bacterial adhesion and biofilm formation on the dental material surfaces that has a critical role on the restoration durability [4, 10]. This study aimed to compare the amount of S. mutans adhesion on zirconia, enamel, IPS Empress II, noble alloy, and base-metal alloy.
2. Materials and Methods
Fifty specimens (5 mm diameter disk with 1 mm thickness) were prepared (10 for each material; zirconia, enamel, IPS Empress II, noble alloy, and base-metal). Zirconia and IPS-Empress specimens were prepared from Ivoclar Vivadent, Schaan, Liechtenstein dental Company by milling. The molds with the mentioned dimension were waxed up and casted with base metal (Ni-Cr-T3, VeraBond, Super Cast) and noble alloy (X33Nourafranco; Switzerland-Italy Inc, Italy). Enamel was regarded as reference for evaluating the bacterial adhesion in vitro environment.
The enamel samples were prepared from recently extracted third molars. Zirconia and IPS-Empress were polished for 30 seconds by the fine 46-µm Diamond Rotary Cutting Instrument (DRCI) and then with extra fine 25-µm DRCI, and finally glazed. The semiprecious and base-metal specimens were polished by Eve (DIAPOl, diamond polishers, Ernst Vetter GmbH Untere Felsentre. 29D-75180 Pforzheim Germany). After preparing the specimens, their surface roughness was measured by using a surface profilometer (Mitutoyo surftest 301, Mitutoyo corporation, Kanagawa, Japan) with a standard cutoff of 0.8 mm and a stylus speed of 0.1 mm/s.
S. mutans ATCC1683 was used in this investigation (American Type Culture Collection). The bacteria obtained from stock was plated on Columbia agar (10455: Merck KGaA) and incubated at 37°C in 10% CO2 atmosphere for 24 hours. Then, the bacteria was obtained from cultures and transferred into tubes that contained BHI (Brain-heat infusion, 10493, Merck KGaA) and incubated at 37°C in 10% CO2 atmosphere for 18 hours. The tube contents were centrifuged and mixed for 5 minutes. Bacterial suspension was concentrated as 1.5×108 bacteria/mL (0.5 McFarland Standard). Before bacterial adhesion, samples were cleaned by ultrasonic device (Mini Sono Cleaner CA 1470, KaijoDenki Co. Ltd., Tokyo, Japan) for 15 minutes and were then sterilized by placing in autoclave at 1210C for 30 minutes.
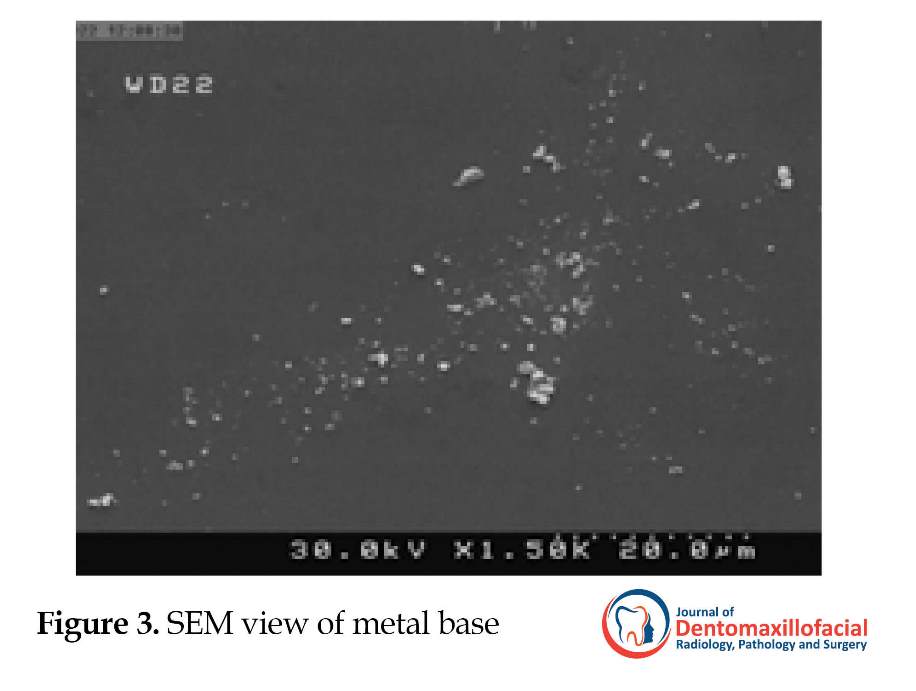
The specimens (10 for each material) were then placed in individual sterile tubes containing 0.5 mL physiologic serum and 0.5 mL bacterial suspension with 0.5 McFarland standard concentration and 1 mL sterile artificial saliva (Hypozalix; BIOCODEX Inc., France) for 10 minutes. The samples were rinsed with 1 mL sterile normal saline and placed on the plates containing solid blood agar culture media and incubated at 37◦C for 48 hours. The estimation of bacteria count on the disk surfaces was examined under light microscope by an observer who was blind about the investigation. In order to evaluate the samples under Scanning Electron Microscope (SEM) (with FE SEM Hitachi scanner) the disks were covered by 10-nm gold thickness with DC sputtering and bacteria colonies around each sample were counted by an experienced operator. For each material, images under different magnification in JPEG format were taken (Figures 1, 2, 3, 4, and 5).
The collected data for adhesion and micro-strength were evaluated by 1-way analysis of variance (ANOVA) and post-hoc Tukey test. Since the dependent variable was quantitative and the normality was confirmed by Kolmogorov-Smirnov test, we used ANOVA model. P<0.05 was considered statistically significant.
3. Results
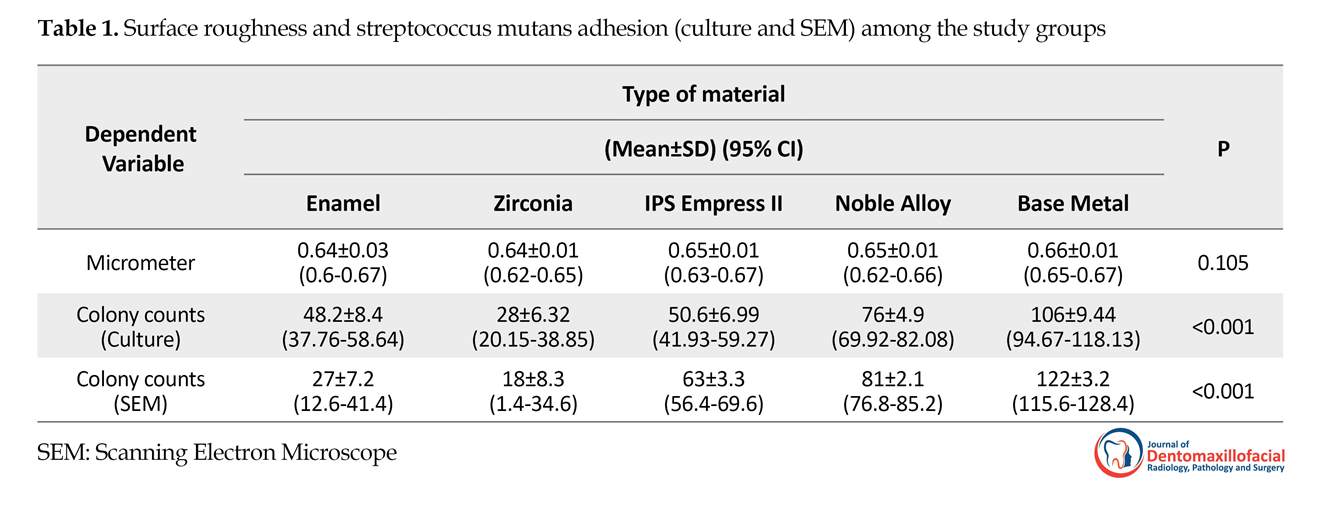
The present study aimed to compare the adhesion of S. mutans on zirconia, base-metal alloy, noble alloy, IPS Empress, and enamel. Results are summarized in Tables 1 and 2. According to Table 1, there was no significant difference among study groups regarding their surface roughness (P=0.105), but their adhesion values showed statistically significant differences (P<0.001).
The lowest adhesion value was obtained in zirconia group (28±6.32) and the highest adhesion value was recorded in base-metal group (106.4±9.44). The results of one-way ANOVA indicated that the bacterial adhesion values varied significantly among 5 groups (P<0.001). The results of Tukey test indicated significant difference in pair comparison of the specimens except the IPS Empress II with enamel group (P=0.985).
4. Discussion
Bacterial plaque formation on tooth surfaces or restorative materials on tooth structures are the major causes of dental diseases. The study of plaque formation and factors affecting it like physical and chemical properties of tooth and restorative materials have an important role in preventing caries [3]. Evaluation of plaque adhesion on different restorative materials is important, as bacterial plaque is one of the major etiologic factors in carries, periodontitis and peri-implantitis [15].
The influence of surface roughness on bacterial adhesion is complicated. Based on the studies conducted on surface roughness and its effects on bacterial adhesion, bacterial accumulation on polished surfaces is more than rough surfaces. According to Bollen et al. the effect of surface roughness on adhesion has a certain threshold; the surface roughness below 0.2 micron has no significant effect on the quantity and quality of bacIn the present study, for matching the investigation with the clinical condition, each study group was polished (except the enamel) with the conventional techniques. The surface roughness did not differ significantly in the study groups. In fact, the significant differences observed in the adhesion between groups indicated the effect of the type of material on adhesion. Because of the hydrophobic properties of S. mutans, its adhesion to hydrophobic surfaces was more. Coating the substrate surface with saliva leads to decreasing the contact angle and therefore becoming the substrate hydrophilic and reducing the adhesion values [16, 17].
Microbial adhesion is the initial stage of colonization and biofilm formation in the mouth environment [1, 2]. After biofilm formation, dental plaque forms on the tooth surfaces and intraoral restorations. Dental plaque is the common reason of caries and oral cavity disease such as gingivitis, periodontitis and peri-implantitis [3]. The contributing factors for bacterial adhesion to restorative surfaces consist of surface substance, electrical charge of microbial particles and their antibacterial effect, surface roughness and its hydrophilic and hydrophobic properties, and the surface proteins of the bacterial membrane [3].
Studies have already been conducted on microbial adhesion to enamel, composite, amalgam, ceramic and metal surfaces [4-8]. Although comprehensive investigations about bacterial adhesion to restorative surfaces have been performed, the current studies reveal a difference in surface properties and bacterial adhesion to restorative surfaces [9-12]. Among the bacteria that are effective on adhesion and formation of the dental plaque, Streptococcus Mutans (S. mutans) is the pioneer and has a critical role in caries and periodontal disease. Therefore, evaluation of S. mutans and its colonization on the restorative materials plays a special role on the success of restorations [4, 10, 13].
Mechanical properties, chemical stability and esthetics are the reasons for the selection of the restorative materials [3, 14]. The use of all ceramic restorations, especially zirconia has increased in the past few years because of their excellent esthetic, high strength and biocompatibility [3, 10]. Up to now, a large number of in vitro and in vivo investigations on restorative materials with respect to their mechanical properties, chemical stability and toxicity have been conducted; however, most studies overlooked the topic of bacterial adhesion and biofilm formation on the dental material surfaces that has a critical role on the restoration durability [4, 10]. This study aimed to compare the amount of S. mutans adhesion on zirconia, enamel, IPS Empress II, noble alloy, and base-metal alloy.
2. Materials and Methods
Fifty specimens (5 mm diameter disk with 1 mm thickness) were prepared (10 for each material; zirconia, enamel, IPS Empress II, noble alloy, and base-metal). Zirconia and IPS-Empress specimens were prepared from Ivoclar Vivadent, Schaan, Liechtenstein dental Company by milling. The molds with the mentioned dimension were waxed up and casted with base metal (Ni-Cr-T3, VeraBond, Super Cast) and noble alloy (X33Nourafranco; Switzerland-Italy Inc, Italy). Enamel was regarded as reference for evaluating the bacterial adhesion in vitro environment.
The enamel samples were prepared from recently extracted third molars. Zirconia and IPS-Empress were polished for 30 seconds by the fine 46-µm Diamond Rotary Cutting Instrument (DRCI) and then with extra fine 25-µm DRCI, and finally glazed. The semiprecious and base-metal specimens were polished by Eve (DIAPOl, diamond polishers, Ernst Vetter GmbH Untere Felsentre. 29D-75180 Pforzheim Germany). After preparing the specimens, their surface roughness was measured by using a surface profilometer (Mitutoyo surftest 301, Mitutoyo corporation, Kanagawa, Japan) with a standard cutoff of 0.8 mm and a stylus speed of 0.1 mm/s.
S. mutans ATCC1683 was used in this investigation (American Type Culture Collection). The bacteria obtained from stock was plated on Columbia agar (10455: Merck KGaA) and incubated at 37°C in 10% CO2 atmosphere for 24 hours. Then, the bacteria was obtained from cultures and transferred into tubes that contained BHI (Brain-heat infusion, 10493, Merck KGaA) and incubated at 37°C in 10% CO2 atmosphere for 18 hours. The tube contents were centrifuged and mixed for 5 minutes. Bacterial suspension was concentrated as 1.5×108 bacteria/mL (0.5 McFarland Standard). Before bacterial adhesion, samples were cleaned by ultrasonic device (Mini Sono Cleaner CA 1470, KaijoDenki Co. Ltd., Tokyo, Japan) for 15 minutes and were then sterilized by placing in autoclave at 1210C for 30 minutes.
The specimens (10 for each material) were then placed in individual sterile tubes containing 0.5 mL physiologic serum and 0.5 mL bacterial suspension with 0.5 McFarland standard concentration and 1 mL sterile artificial saliva (Hypozalix; BIOCODEX Inc., France) for 10 minutes. The samples were rinsed with 1 mL sterile normal saline and placed on the plates containing solid blood agar culture media and incubated at 37◦C for 48 hours. The estimation of bacteria count on the disk surfaces was examined under light microscope by an observer who was blind about the investigation. In order to evaluate the samples under Scanning Electron Microscope (SEM) (with FE SEM Hitachi scanner) the disks were covered by 10-nm gold thickness with DC sputtering and bacteria colonies around each sample were counted by an experienced operator. For each material, images under different magnification in JPEG format were taken (Figures 1, 2, 3, 4, and 5).
The collected data for adhesion and micro-strength were evaluated by 1-way analysis of variance (ANOVA) and post-hoc Tukey test. Since the dependent variable was quantitative and the normality was confirmed by Kolmogorov-Smirnov test, we used ANOVA model. P<0.05 was considered statistically significant.
3. Results
The present study aimed to compare the adhesion of S. mutans on zirconia, base-metal alloy, noble alloy, IPS Empress, and enamel. Results are summarized in Tables 1 and 2. According to Table 1, there was no significant difference among study groups regarding their surface roughness (P=0.105), but their adhesion values showed statistically significant differences (P<0.001).
The lowest adhesion value was obtained in zirconia group (28±6.32) and the highest adhesion value was recorded in base-metal group (106.4±9.44). The results of one-way ANOVA indicated that the bacterial adhesion values varied significantly among 5 groups (P<0.001). The results of Tukey test indicated significant difference in pair comparison of the specimens except the IPS Empress II with enamel group (P=0.985).
4. Discussion
Bacterial plaque formation on tooth surfaces or restorative materials on tooth structures are the major causes of dental diseases. The study of plaque formation and factors affecting it like physical and chemical properties of tooth and restorative materials have an important role in preventing caries [3]. Evaluation of plaque adhesion on different restorative materials is important, as bacterial plaque is one of the major etiologic factors in carries, periodontitis and peri-implantitis [15].
The influence of surface roughness on bacterial adhesion is complicated. Based on the studies conducted on surface roughness and its effects on bacterial adhesion, bacterial accumulation on polished surfaces is more than rough surfaces. According to Bollen et al. the effect of surface roughness on adhesion has a certain threshold; the surface roughness below 0.2 micron has no significant effect on the quantity and quality of bacIn the present study, for matching the investigation with the clinical condition, each study group was polished (except the enamel) with the conventional techniques. The surface roughness did not differ significantly in the study groups. In fact, the significant differences observed in the adhesion between groups indicated the effect of the type of material on adhesion. Because of the hydrophobic properties of S. mutans, its adhesion to hydrophobic surfaces was more. Coating the substrate surface with saliva leads to decreasing the contact angle and therefore becoming the substrate hydrophilic and reducing the adhesion values [16, 17].
The hydrophilic properties of the surfaces is effective on the initial nonspecific bacterial adhesion and specific agents on the cellular wall of the bacteria have greater influence on the adhesion [18]. S. mutans adheres to pellicle on the substrate and other bacteria by formation of extracellular glucan from sucrose and adhesion to this polysaccharide by glucan binding proteins [19]. S. mutans can adhere to the saliva mucin by its surface enolase protein [20].
According to this investigation, the adhesion of S. mutans had significant differences among the study groups. The least adhesion was observed with zirconia, tooth enamel, IPS Empress, noble alloy, and the highest level of adhesion was seen to base-metal alloys. In the present study, the differences in the study groups can be directly correlated to the substrate composition that can influence the initial bacterial adhesion and indirectly by differences in the pellicle layer formed on the substrate (specially based on its mucin). In the research studies, different methods like culture and colony numeration under light microscope, observation of bacteria adherence to surface by SEM and measuring the light generated by the bacteria through spectrofluorometer were used [10, 21-23]. In this investigation for evaluating the amount of S. mutans adhesion, culture and colony numeration under light microscope and SEM method were used and the SEM results confirmed the culture results.
The comparison of the studies was difficult because of the different techniques used for evaluating the bacteria adhesion, not using enamel as the control group in some studies and the different surface roughness in studied materials. The use of enamel as the control group results in better comparison between different materials that are not compared with each other in a single investigation. Therefore in conducting this study, enamel was used as the control group [10].
For eliminating the effect of surface roughness on the degree of bacteria adhesion, the disks first went under polishing and then the surface roughness of four study groups and enamel was evaluated by profilometer and the results indicated no statistically significant difference in the paired comparison of the groups. In the current study, the adhesion of S. mutans to the samples made of zirconia ceramics was lower than IPS-Empress which was also statistically significant.
In Kantorski et al. investigation on two types of ceramics; feldespatics and lucite/feldespatics in comparison to enamel, the adhesion of S. mutans to specimens formed by enamel was more than porcelain [23]. In this study the surface roughness of enamel was more than ceramics. Based on the results of our study, there is no significant difference in surface roughness of the studied materials and enamel and adhesion to enamel was higher than zirconia but less than IPS-Empress. In the current study, there was no significant difference between zirconia and IPS-Empress surface roughness but bacterial adhesion to zirconia was lower. In Bremers investigation which was similar to our study with respect to surface roughness, the results were in agreement with our study [24].
According to this study, the adhesion of S. mutans to enamel was less than noble (X33) and base -metal (super one) which is similar to Sardin et al. results. In Sardin et al. investigation, by polishing the surfaces and creating similar surface roughness in the range of 0.1 micron, the influence of surface roughness on bacteria adhesion was eliminated and in comparison with the four alloys, noble and a base-metal alloy (Ni-Cr), the lowest adhesion was observed in enamel [25].
In comparison between noble and base-metal alloys in the current study, the adhesion of S. mutans to noble alloy was lower which is in contrast with Grivet et al. findings. In Grivet et al. study on four precious alloys and base-metal, although the surface roughness of five study groups ranged from 0.02 to 0.06 µm and the influence of surface roughness values on the bacterial adhesion was not statistically significant, the amount of S. mutans adherence was higher in precious group than in base-metal. This difference may be because of the surface free energy of the tested materials, selection of materials from different companies and differences in the methods of investigation [26].
5. Conclusion
Different restorative materials have different bacteria adhesion. Zirconia had the lowest adhesion among the tested materials and the highest adhesion was seen in the base-metal. Based on the findings of the current study, the use of restorative ceramics, including zirconia is a better choice in patients with poor oral hygiene and those who are susceptible to periodontal disease.
Limitation of our study was related to the evaluation of one type of oral bacteria. More studies are recommended on other bacteria and using dynamic procedures like flow chamber to simulate oral environment.
Ethical Considerations
Compliance with ethical guidelines
There was no ethical considerations to be considered in this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
- Dankert J. Biomedical polymers: bacterial adhesion, colonization and infection. CRC Critical Reviews in Biocompatibility. 1986; 2:219-301.
- Hori K, Matsumoto S. Bacterial adhesion: From mechanism to control. Journal of Biomechanical Engineering. 2010; 48(3):424-34. [DOI:10.1016/j.bej.2009.11.014]
- Hannig M. Transmission electron microscopic study of in vivo pellicle formation on dental restorative materials. European Journal of Oral Sciences. 1997; 105(5 Pt 1):422-33. [DOI:10.1111/j.1600-0722.1997.tb02139.x] [PMID]
- Hannig M. Transmission electron microscopy of early plaque formation on dental materials in vivo. European Journal of Oral Sciences. 1999; 107(1):55-64. [DOI:10.1046/j.0909-8836.1999.eos107109.x] [PMID]
- Guggenheim B, Giertsen E, Schüpbach P, Shapiro S. Validation of an in vitro biofilm model of supragingival plaque. Journal of Dental Research. 2001; 80(1):363-70. [DOI:10.1177/00220345010800011201] [PMID]
- Dezelic T, Schmidlin PR. Multi-species biofilm formation on dental materials and an adhesive patch. Oral Health & Preventive Dentistry. 2009; 7(1):47-53. [PMID]
- Carlen A, Nikdel K, Wennerberg A, Holmberg K, Olsson J. Surface characteristics and in vitro biofilm formation on glass ionomer and composite resin. Biomaterials. 2001; 22(5):481-7. [DOI:10.1016/S0142-9612(00)00204-0]
- Carlen A, Börjesson AC, Nikdel K, Olsson J. Composition of pellicles formed in vivo on tooth surfaces in different parts of the dentition, and in vitro on hydroxyapatite. Caries Research. 1998; 32(6):447-55 [DOI:10.1159/000016486] [PMID]
- Suljak J, Reid G, Wood S, McConnell R, Van der Mei H, Busscher H. Bacterial adhesion to dental amalgam and three resin composites. Journal of Dentistry. 1995; 23(3):171-6. [DOI:10.1016/0300-5712(95)93575-M]
- Meier R, Hauser Gerspach I, Lüthy H, Meyer J. Adhesion of oral streptococci to all-ceramics dental restorative materials in vitro. Journal of Materials Science: Materials in Medicine. 2008; 19(10):3249-53. [DOI:10.1007/s10856-008-3457-7] [PMID]
- Prati C, Fava F, Di Gioia D, Selighini M, Pashley DH. Antibacterial effectiveness of dentin bonding systems. Dental Materials. 1993; 9(6):338-43. [DOI:10.1016/0109-5641(93)90053-S]
- Palenik C, Behnen M, Setcos J, Miller C. Inhibition of microbial adherence and growth by various glass ionomers in vitro. Dental Materials. 1992; 8(1):16-20. [DOI:10.1016/0109-5641(92)90047-G]
- Satou J, Fukunaga A, Satou N, Shintani H, Okuda K. Streptococcal adherence on various restorative materials. Dental Materials. 1988; 67(3):588-91. [DOI:10.1177/00220345880670031301] [PMID]
- Lee BH, Do Kim Y, Shin JH, Hwan Lee K. Surface modification by alkali and heat treatments in titanium alloys. Journal of Biomedical Materials Research. 2002; 61(3):466-73. [DOI:10.1002/jbm.10190] [PMID]
- Della Bona A, Kelly JR. The clinical success of all-ceramic restorations. Journal of the American Dental Association. 2008; 139(Suppl 4):8S-13S. [DOI:10.14219/jada.archive.2008.0361] [PMID]
- Bollen CM, Papaioanno W, Van Eldere J, Schepers E, Quirynen M, Van Steenberghe D. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clinical Oral Implants Research. 1996; 7(3):201-11. [DOI:10.1034/j.1600-0501.1996.070302.x] [PMID]
- Quirynen M, Bollen C. The influence of surface roughness and surface‐free energy on supra‐and subgingival plaque formation in man: A review of the literature. Journal of Clinical Periodontology. 1995; 22(1):1-14. [DOI:10.1111/j.1600-051X.1995.tb01765.x] [PMID]
- Teughels W, Van Assche N, Sliepen I, Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clinical Oral Implants Research. 2006; 17(Suppl 2):68-81. [DOI:10.1111/j.1600-0501.2006.01353.x] [PMID]
- Banas J, Vickerman M. Glucan-binding proteins of the oral streptococci. Critical Reviews in Oral Biology & Medicine. 2003; 14(2):89-99. [DOI:10.1177/154411130301400203] [PMID]
- Egawa M, Miura T, Kato T, Saito A, Yoshinari M. In vitro adherence of periodontopathic bacteria to zirconia and titanium surfaces. Dental Materials Journal. 2013; 32(1):101-6. [DOI:10.4012/dmj.2012-156] [PMID]
- Rosentritt M, Behr M, Bürgers R, Feilzer AJ, Hahnel S. In vitro adherence of oral streptococci to zirconia core and veneering glass‐ceramics. Journal of Biomedical Materials Research Part B. 2009; 91(1):257-63. [DOI:10.1002/jbm.b.31397] [PMID]
- Scarano A, Piattelli M, Caputi S, Favero GA, Piattelli A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. Journal of Periodontology. 2004; 75(2):292-6. [DOI:10.1902/jop.2004.75.2.292] [PMID]
- Kantorski KZ, Scotti R, Valandro LF, Bottino MA, Koga Ito CY, Jorge AO. Adherence of streptococcus mutans to uncoated and saliva-coated glass-ceramics and composites. General Dentistry. 2008; 56(7):740-7. [PMID]
- Bremer F, Grade S, Kohorst P, Stiesch M. In vivo biofilm formation on different dental ceramics. Quintessence Publishing: Quintessence International. 2011; 42(7):565-74.
- Sardin S, Morrier JJ, Benay G, Barsotti O. In vitro streptococcal adherence on prosthetic and implant materials. Interactions with physicochemical surface properties. Journal of Oral Rehabilitation. 2004; 31(2):140-8. [DOI:10.1046/j.0305-182X.2003.01136.x] [PMID]
- Grivet M, Morrier JJ, Benay G, Barsotti O. Effect of hydrophobicity on in vitro streptococcal adhesion to dental alloys. Journal of Materials Science: Materials in Medicine. 2000; 11(10):637-42. [DOI:10.1023/A:1008913915399]
Type of Study: Original article |
Subject:
Pathology
Received: 2017/10/10 | Accepted: 2018/01/25 | Published: 2018/03/1
Received: 2017/10/10 | Accepted: 2018/01/25 | Published: 2018/03/1
References
1. Dankert J. Biomedical polymers: bacterial adhesion, colonization and infection. CRC Critical Reviews in Biocompatibility. 1986; 2:219-301.
2. Hori K, Matsumoto S. Bacterial adhesion: From mechanism to control. Journal of Biomechanical Engineering. 2010; 48(3):424-34. [DOI:10.1016/j.bej.2009.11.014] [DOI:10.1016/j.bej.2009.11.014]
3. Hannig M. Transmission electron microscopic study of in vivo pellicle formation on dental restorative materials. European Journal of Oral Sciences. 1997; 105(5 Pt 1):422-33. [DOI:10.1111/j.1600-0722.1997.tb02139.x] [PMID] [DOI:10.1111/j.1600-0722.1997.tb02139.x]
4. Hannig M. Transmission electron microscopy of early plaque formation on dental materials in vivo. European Journal of Oral Sciences. 1999; 107(1):55-64. [DOI:10.1046/j.0909-8836.1999.eos107109.x] [PMID] [DOI:10.1046/j.0909-8836.1999.eos107109.x]
5. Guggenheim B, Giertsen E, Schüpbach P, Shapiro S. Validation of an in vitro biofilm model of supragingival plaque. Journal of Dental Research. 2001; 80(1):363-70. [DOI:10.1177/00220345010800011201] [PMID] [DOI:10.1177/00220345010800011201]
6. Dezelic T, Schmidlin PR. Multi-species biofilm formation on dental materials and an adhesive patch. Oral Health & Preventive Dentistry. 2009; 7(1):47-53. [PMID] [PMID]
7. Carlen A, Nikdel K, Wennerberg A, Holmberg K, Olsson J. Surface characteristics and in vitro biofilm formation on glass ionomer and composite resin. Biomaterials. 2001; 22(5):481-7. [DOI:10.1016/S0142-9612(00)00204-0] [DOI:10.1016/S0142-9612(00)00204-0]
8. Carlen A, Börjesson AC, Nikdel K, Olsson J. Composition of pellicles formed in vivo on tooth surfaces in different parts of the dentition, and in vitro on hydroxyapatite. Caries Research. 1998; 32(6):447-55 [DOI:10.1159/000016486] [PMID] [DOI:10.1159/000016486]
9. Suljak J, Reid G, Wood S, McConnell R, Van der Mei H, Busscher H. Bacterial adhesion to dental amalgam and three resin composites. Journal of Dentistry. 1995; 23(3):171-6. [DOI:10.1016/0300-5712(95)93575-M] [DOI:10.1016/0300-5712(95)93575-M]
10. Meier R, Hauser Gerspach I, Lüthy H, Meyer J. Adhesion of oral streptococci to all-ceramics dental restorative materials in vitro. Journal of Materials Science: Materials in Medicine. 2008; 19(10):3249-53. [DOI:10.1007/s10856-008-3457-7] [PMID] [DOI:10.1007/s10856-008-3457-7]
11. Prati C, Fava F, Di Gioia D, Selighini M, Pashley DH. Antibacterial effectiveness of dentin bonding systems. Dental Materials. 1993; 9(6):338-43. [DOI:10.1016/0109-5641(93)90053-S] [DOI:10.1016/0109-5641(93)90053-S]
12. Palenik C, Behnen M, Setcos J, Miller C. Inhibition of microbial adherence and growth by various glass ionomers in vitro. Dental Materials. 1992; 8(1):16-20. [DOI:10.1016/0109-5641(92)90047-G] [DOI:10.1016/0109-5641(92)90047-G]
13. Satou J, Fukunaga A, Satou N, Shintani H, Okuda K. Streptococcal adherence on various restorative materials. Dental Materials. 1988; 67(3):588-91. [DOI:10.1177/00220345880670031301] [PMID] [DOI:10.1177/00220345880670031301]
14. Lee BH, Do Kim Y, Shin JH, Hwan Lee K. Surface modification by alkali and heat treatments in titanium alloys. Journal of Biomedical Materials Research. 2002; 61(3):466-73. [DOI:10.1002/jbm.10190] [PMID] [DOI:10.1002/jbm.10190]
15. Della Bona A, Kelly JR. The clinical success of all-ceramic restorations. Journal of the American Dental Association. 2008; 139(Suppl 4):8S-13S. [DOI:10.14219/jada.archive.2008.0361] [PMID] [DOI:10.14219/jada.archive.2008.0361]
16. Bollen CM, Papaioanno W, Van Eldere J, Schepers E, Quirynen M, Van Steenberghe D. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clinical Oral Implants Research. 1996; 7(3):201-11. [DOI:10.1034/j.1600-0501.1996.070302.x] [PMID] [DOI:10.1034/j.1600-0501.1996.070302.x]
17. Quirynen M, Bollen C. The influence of surface roughness and surface‐free energy on supra‐and subgingival plaque formation in man: A review of the literature. Journal of Clinical Periodontology. 1995; 22(1):1-14. [DOI:10.1111/j.1600-051X.1995.tb01765.x] [PMID] [DOI:10.1111/j.1600-051X.1995.tb01765.x]
18. Teughels W, Van Assche N, Sliepen I, Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clinical Oral Implants Research. 2006; 17(Suppl 2):68-81. [DOI:10.1111/j.1600-0501.2006.01353.x] [PMID] [DOI:10.1111/j.1600-0501.2006.01353.x]
19. Banas J, Vickerman M. Glucan-binding proteins of the oral streptococci. Critical Reviews in Oral Biology & Medicine. 2003; 14(2):89-99. [DOI:10.1177/154411130301400203] [PMID] [DOI:10.1177/154411130301400203]
20. Egawa M, Miura T, Kato T, Saito A, Yoshinari M. In vitro adherence of periodontopathic bacteria to zirconia and titanium surfaces. Dental Materials Journal. 2013; 32(1):101-6. [DOI:10.4012/dmj.2012-156] [PMID] [DOI:10.4012/dmj.2012-156]
21. Rosentritt M, Behr M, Bürgers R, Feilzer AJ, Hahnel S. In vitro adherence of oral streptococci to zirconia core and veneering glass‐ceramics. Journal of Biomedical Materials Research Part B. 2009; 91(1):257-63. [DOI:10.1002/jbm.b.31397] [PMID] [DOI:10.1002/jbm.b.31397]
22. Scarano A, Piattelli M, Caputi S, Favero GA, Piattelli A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. Journal of Periodontology. 2004; 75(2):292-6. [DOI:10.1902/jop.2004.75.2.292] [PMID] [DOI:10.1902/jop.2004.75.2.292]
23. Kantorski KZ, Scotti R, Valandro LF, Bottino MA, Koga Ito CY, Jorge AO. Adherence of streptococcus mutans to uncoated and saliva-coated glass-ceramics and composites. General Dentistry. 2008; 56(7):740-7. [PMID] [PMID]
24. Bremer F, Grade S, Kohorst P, Stiesch M. In vivo biofilm formation on different dental ceramics. Quintessence Publishing: Quintessence International. 2011; 42(7):565-74.
25. Sardin S, Morrier JJ, Benay G, Barsotti O. In vitro streptococcal adherence on prosthetic and implant materials. Interactions with physicochemical surface properties. Journal of Oral Rehabilitation. 2004; 31(2):140-8. [DOI:10.1046/j.0305-182X.2003.01136.x] [PMID] [DOI:10.1046/j.0305-182X.2003.01136.x]
26. Grivet M, Morrier JJ, Benay G, Barsotti O. Effect of hydrophobicity on in vitro streptococcal adhesion to dental alloys. Journal of Materials Science: Materials in Medicine. 2000; 11(10):637-42. [DOI:10.1023/A:1008913915399] [DOI:10.1023/A:1008913915399]
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |