Sat, Apr 27, 2024
Volume 7, Issue 1 (3-2018)
2018, 7(1): 37-42 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Aminishakib P, Kashefi M, Moradi Ghahdarijani B, Yazdani F, Nafarzadeh S, Gholami A, et al . Neoangiogenesis in Benign and Malignant Salivary Gland Tumors. Journal title 2018; 7 (1) :37-42
URL: http://3dj.gums.ac.ir/article-1-299-en.html
URL: http://3dj.gums.ac.ir/article-1-299-en.html
Pouyan Aminishakib1 

 , Mohammad Kashefi2
, Mohammad Kashefi2 
 , Behrooz Moradi Ghahdarijani3
, Behrooz Moradi Ghahdarijani3 
 , Farzad Yazdani4
, Farzad Yazdani4 
 , Shima Nafarzadeh5
, Shima Nafarzadeh5 

 , Azadeh Gholami5
, Azadeh Gholami5 
 , Ali Bijani6
, Ali Bijani6 

 , Sara Mehrabi *
, Sara Mehrabi * 

 7
7


 , Mohammad Kashefi2
, Mohammad Kashefi2 
 , Behrooz Moradi Ghahdarijani3
, Behrooz Moradi Ghahdarijani3 
 , Farzad Yazdani4
, Farzad Yazdani4 
 , Shima Nafarzadeh5
, Shima Nafarzadeh5 

 , Azadeh Gholami5
, Azadeh Gholami5 
 , Ali Bijani6
, Ali Bijani6 

 , Sara Mehrabi *
, Sara Mehrabi * 

 7
7
1- Department of Oral and Maxillofacial Pathology, School of Dentistry, Tehran University of Medical Sciences, Tehran, Iran.
2- Dental Material Research Center, School of Dentistry, Babol University of Medical Sciences, Babol, Iran.
3- School of Dentistry, Babol University of Medical Sciences, Babol, Iran.
4- Department of Pathology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
5- Department of Oral and Maxillofacial Pathology, School of Dentistry, Babol University of Medical Sciences, Babol, Iran.
6- Social Determinant of Health Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran.
7- Department of Oral and Maxillofacial Pathology, School of Dentistry, Zanjan University of Medical Sciences, Zanjan, Iran. , dr.mehrabi@zums.ac.ir
2- Dental Material Research Center, School of Dentistry, Babol University of Medical Sciences, Babol, Iran.
3- School of Dentistry, Babol University of Medical Sciences, Babol, Iran.
4- Department of Pathology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
5- Department of Oral and Maxillofacial Pathology, School of Dentistry, Babol University of Medical Sciences, Babol, Iran.
6- Social Determinant of Health Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran.
7- Department of Oral and Maxillofacial Pathology, School of Dentistry, Zanjan University of Medical Sciences, Zanjan, Iran. , dr.mehrabi@zums.ac.ir
Full-Text [PDF 1959 kb]
(654 Downloads)
| Abstract (HTML) (4078 Views)
In the present study, MVD was assessed in the intratumoral region because the highest level of angiogenesis due to oxygen and nutrient demands is in this region [2]; however, peri-tumoral vessels more commonly reflect the activities and reactions of the stroma and matrix surrounding the tumor, i.e. the invasiveness of the tumor. CD105 expression was not or minimally observed in normal salivary gland tissues. Moreover, its expression in PA lesions, as the major benign salivary gland neoplasm, was not different than normal tissue. On the other hand, MEC and AdCC demonstrated significantly higher CD105 expressions than PA and normal tissues in our study, along with some other studies [2], which can probably be attributed to their more progressive and aggressive nature. However, this is not always the case, as some malignant tumors have not demonstrated CD105 expression1 probably because of lower oxygen and nutrient needs, resulting in less apparent angiogenesis; this particularly holds true in case of AdCC, which can continue its metabolic activities without high oxygen levels. Nevertheless, evidence of angiogenesis can translate into more aggressive behavior of the tumor [1].
An interesting finding in our study is the significantly higher expression level of CD105 in MEC than in AdCC, which is in accordance with other studies showing the highest angiogenic activity in MEC compared to other benign and malignant salivary gland neoplasms [1, 24]. One can attribute it to the absence of myoepithelial cells in MEC, which are potentially anti-angiogenic [24, 25]. In the absence of proteases and inhibitors of angiogenesis, MEC with its high oxygen demand (due to inability to use glycolysis for its energy source) demonstrates a high MVD index [26].
Sustained hypoxia is toxic to both normal and tumoral cells and usually leads to secretion of angiogenic stimulants such as TGFB by the tumor-related inflammatory cells [27], which in turn upregulates CD105 expression. However, contrary to this theory, tumoral cells might acquire a genetic or epigenetic adaptive advantage in the hypoxic environment, leading to the ability to survive and even proliferate irrespective of the surrounding oxygen level and thus overcoming the need for angiogenesis, which in turn increases their invasion and metastasis potential [28]. Our findings, nevertheless, support the assessment of vascularization and neoangiogenesis as a marker to differentiate malignant from benign neoplasms of the salivary glands.
5. Conclusion
In conclusion, this study showed that CD105 expression and MVD are significantly higher in malignant tumors of the salivary glands than their benign counterparts and normal tissue. This further highlights the role of neoangiogenesis in the pathophysiology of these tumors. We suggest that the association of CD105 expression in malignant salivary gland tumors and disease prognosis be investigated as a future research perspective.
Ethical Considerations
Compliance with ethical guidelines
There is no ethical principle to be considered doing this research.
Funding
This study was funded in part by Babol University of Medical Sciences, and the grant number is 9236710.
Authors contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declare no conflict of interest.
References
Full-Text: (847 Views)
1. Introduction
Salivary gland tumors are among the most challenging topics in oncology because of their high prevalence, diverse histopathology, difficult resectability, and poor response to different treatment modalities. In addition, poor understanding of their underlying biological mechanisms of growth and spread, compared to other neoplasms, have further complicated their clinical management [1].
Mucoepidermoid Carcinoma (MEC) is the most common salivary gland malignancy in children and adults. It demonstrates highly variable clinical behaviors and its pathogenesis is not fully understood. This lack of data regarding factors affecting the prognosis of this tumor needs to be addressed [2, 3]. Although Adenoid Cystic Carcinoma (AdCC) is the most studied salivary gland tumor, it is still associated with high recurrence rates and metastasis [3]. Pleomorphic Adenoma (PA) is another tumor of the major and minor salivary glands, which can be successfully treated surgically because of its benign nature [3].
Tumors have high oxygen and nutrient demands, which often limit their extension. This is why neoangiogenesis, which is referred to the formation of new blood vessels, is a sine qua non for their growth and spread [4]. It can be best described by imbalance between pro-angiogenic and anti-angiogenic triggers by both the normal and tumor cells. Angiogenesis consists of multiple cellular mechanisms such as cell migration, proliferation, differentiation into small vessels, anastomosis, degradation of extracellular matrix, and structural reorganization [5]. It has been demonstrated in many neoplasms in humans, including breast cancer, melanoma, ameloblastoma, as well as lichen planus [6].
The conventional method to assess angiogenesis in solid tumors is quantitative measurement of Micro-Vessel Density (MVD) using Immunohistochemistry (IHC) [7]. CD105, also known as endoglin, is a type 1 transmembrane homodimeric glycoprotein of 180 kDa [8] and serves as a part of the receptor complex for transforming growth factors beta 1 (TGF-B1) and TGF-B3 [9]. It plays a part in fibrogenesis and angiogenesis [10], and is particularly important for proliferation of endothelial cells and stimulating the activation phase of angiogenesis [11]. It is thus widely expressed in newly-formed vessels versus the old vessels [10, 12], and is a reliable marker for neovascularization in solid tumors, superior to CD31 and CD34 in assessing angiogenesis in growing tumors [13].
In this study, we aimed to undertake a comparative assessment of intratumoral Micro-Vessel Density (MVD) using immunohistochemical expression of CD105 biomarker in PA, MEC, AdCC and normal salivary gland tissue.
2. Materials and Methods
Study specimens
In this cross-sectional comparative study, we selected 20 specimens of PA, 20 specimens of MEC, 20 specimens of AdCC, and 10 specimens of normal salivary gland tissue in paraffin blocks retrieved from the archives of the Pathology Department of Tehran Amir A′lam Hospital, which were collected from 2013 to 2015. All specimen slides, stained with hematoxylin and eosin, were reviewed and rechecked by two independent pathologists to confirm the diagnoses.
Immunohistochemistry
Four-micrometer slices were made by a microtome from each paraffin block and were deparaffinized and rehydrated with xylene and alcohol, respectively. Antigen retrieval was then performed using 1 mM Ethylenediaminetetraacetic Acid (EDTA) buffer with a pH of 8. After irrigation with sterile water and 0.5 M Tris Hydrochloride (Tris-HCL) with a pH of 7, internal peroxidase activity was inhibited by applying 3% oxygen peroxide (H2O2) for 20 minutes.
Slices were then irrigated and incubated in endogenous Avidin/Biotin blocking solution for another 20 minutes. After another irrigation with water and buffer, primary antibody (monoclonal mouse CD105 clone SN6h, 1:100, DAKO, Denmark) was applied for 19 hours at 4°C, according to the manufacturer’s instructions. For reaction amplification, secondary antibodies of biotinylated anti-mouse and Dako streptavidin-biotin-peroxidase were used for 30 minutes each. After meticulous irrigation, staining procedure was performed using 3,3’-diaminobenzidine hydrochloride, and 0.02% H2O2, then Mayer’s hematoxylin solution was used for counterstaining. Negative controls were prepared using the same protocol except for the application of primary antibody, while large B-cell lymphoma and tonsillar tissue were used as positive controls.
Quantitative assessment of MVD
Quantitative assessment of mean MVD was performed according to 2002 global consensus [14] as follows: using low magnification (100x) in multiple slices of each specimen, 10 fields with the highest number of intratumoral vascularization were identified and considered as hotspots. Small vessels in each hotspot were then counted using high magnification (400x). Moreover, any single cell or cluster of cells with a brown color with or without an apparent lumen, branching from an adjacent small vessel, tumoral, or connective tissue was counted as a small vessel. Finally, the average of small vessel counts in these 10 fields was calculated for each sample and considered as the corresponding mean MVD.
Statistical analysis
All statistical analyses were carried out in SPSS version 20 (SPSS Inc., Chicago, IL, USA). The Mann-Whitney U test and the Kruskal-Wallis test were used to compare intratumoral mean MVD between groups. Moreover, between-group differences were evaluated using 1-way ANOVA and Tukey post hoc test. Differences with P values less than 0.05 were considered statistically significant.
3. Results
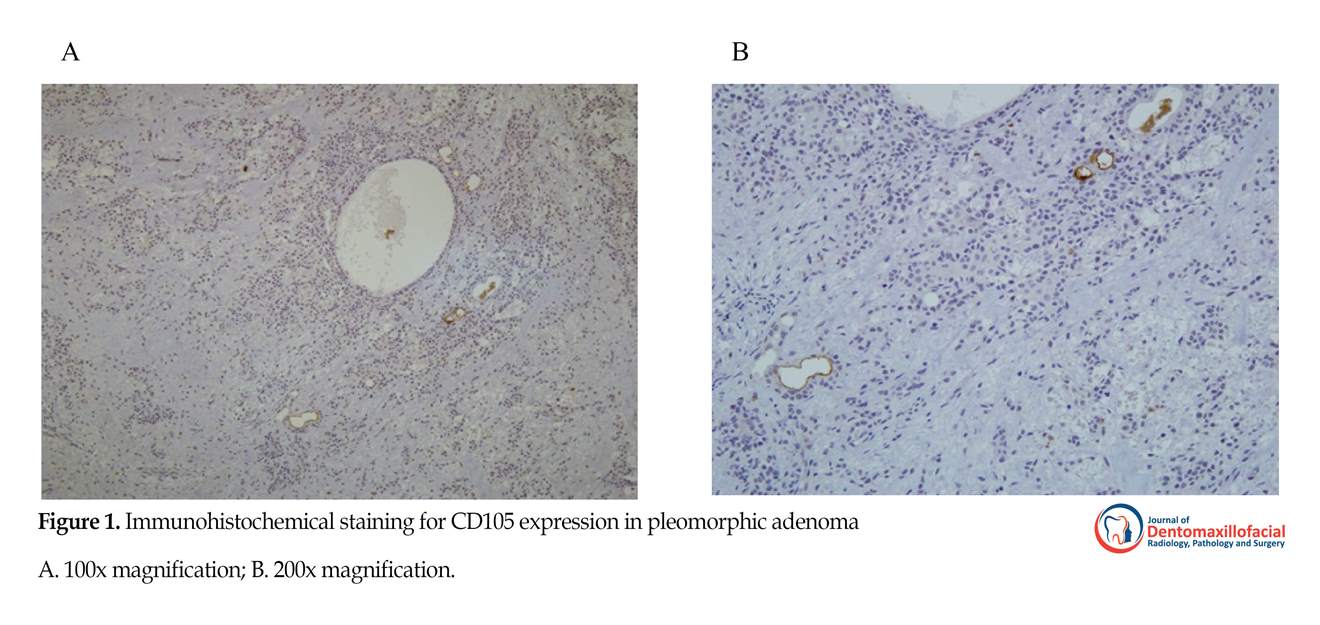
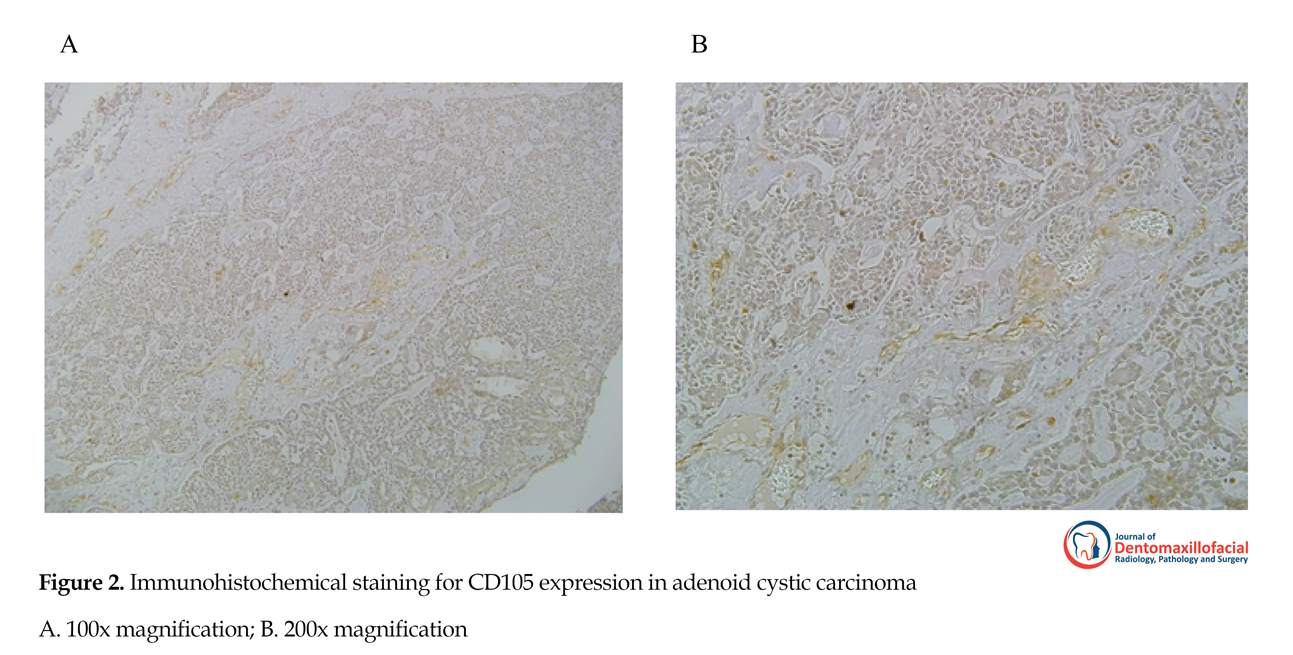
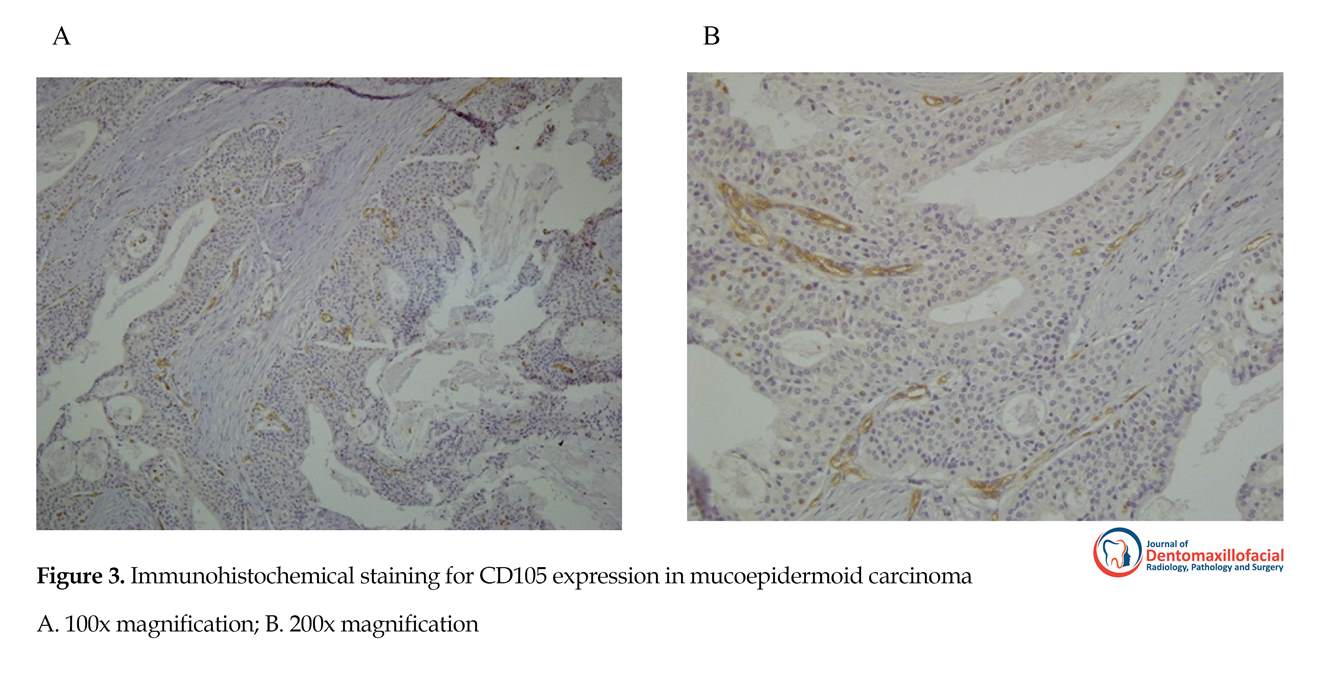
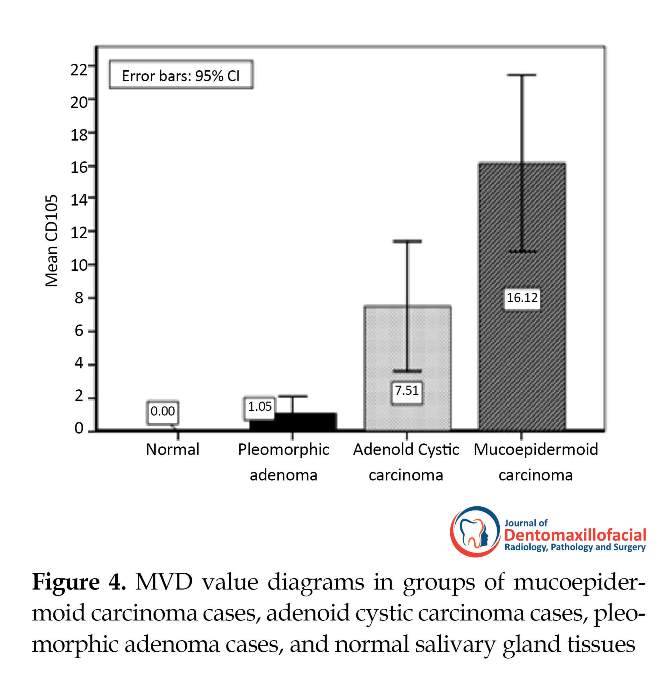
CD105-expressing vessels were rarely seen in normal salivary tissues. Expression of CD105 was demonstrated in tumors as brown cytoplasmic and membranous staining in endothelial cells of small vessels. Significant differences were observed in intratumoral MVD between normal salivary tissue and AdCC (P<0.017) or MEC (P<0.001), while PA was similar to normal salivary gland tissue in this regard. CD105 expression was significantly higher in the malignant lesions of AdCC and MEC compared to the benign PA (P<0.018 and P<0.001, respectively). In addition, MEC had the highest intratumoral MVD among all lesions, even significantly higher than AdCC (P<0.002). CD105 staining density was also the highest in MEC, while PA showed minimal staining. CD105 expressions and MVD in the three tumoral groups are illustrated in Figures 1-4.
4. Discussion
Diverse clinical course has remained the biggest challenge in management of salivary gland tumors [2], which calls for a comprehensive study of their underlying biology. In general, metastasis is the main factor affecting prognosis and mortality in patients with malignant neoplasms. Some evidence suggests the role of angiogenesis in metastasizing malignant tumors [15]. Higher degree of angiogenesis is associated with poorer prognosis in several cancers, including breast, lung, esophagus, colon, and gastric cancer [7, 8, 12], although evidence to the contrary exists [16-19]. This study aimed to assess neoangiogenesis in the most common benign neoplasm of the salivary glands, PA, as well as important malignant salivary gland tumors namely MEC and AdCC.
Folkman was the first to introduce the concept of angiogenesis [20], and Weidner and colleagues proposed a method to evaluate small vessels in tumoral tissue [2]. Pan-endothelial markers such as CD31, CD34, and Von Willebrand Factor (vWF) have been widely used for this matter in normal and tumoral tissues, but each has its own limitations [21, 22]. CD31 stains similarly positive in small and large vessels, as well as some cells in different carcinomas. CD34, on the other hand, can successfully identify active angiogenic cells, but is not specific and stains positive in mesenchymal cells as well. vWF marker sometimes fails to identify small vessels of normal and tumoral tissues, and may also be expressed in lymphatic vessels [13]. This non-specificity could explain the controversial results in studies on the association of angiogenesis and tumor spread. Introduction of new antibodies, such as CD105, has provided new opportunities for the study of neoangiogenesis in tumors, especially regarding endothelial function and growth [23].
Salivary gland tumors are among the most challenging topics in oncology because of their high prevalence, diverse histopathology, difficult resectability, and poor response to different treatment modalities. In addition, poor understanding of their underlying biological mechanisms of growth and spread, compared to other neoplasms, have further complicated their clinical management [1].
Mucoepidermoid Carcinoma (MEC) is the most common salivary gland malignancy in children and adults. It demonstrates highly variable clinical behaviors and its pathogenesis is not fully understood. This lack of data regarding factors affecting the prognosis of this tumor needs to be addressed [2, 3]. Although Adenoid Cystic Carcinoma (AdCC) is the most studied salivary gland tumor, it is still associated with high recurrence rates and metastasis [3]. Pleomorphic Adenoma (PA) is another tumor of the major and minor salivary glands, which can be successfully treated surgically because of its benign nature [3].
Tumors have high oxygen and nutrient demands, which often limit their extension. This is why neoangiogenesis, which is referred to the formation of new blood vessels, is a sine qua non for their growth and spread [4]. It can be best described by imbalance between pro-angiogenic and anti-angiogenic triggers by both the normal and tumor cells. Angiogenesis consists of multiple cellular mechanisms such as cell migration, proliferation, differentiation into small vessels, anastomosis, degradation of extracellular matrix, and structural reorganization [5]. It has been demonstrated in many neoplasms in humans, including breast cancer, melanoma, ameloblastoma, as well as lichen planus [6].
The conventional method to assess angiogenesis in solid tumors is quantitative measurement of Micro-Vessel Density (MVD) using Immunohistochemistry (IHC) [7]. CD105, also known as endoglin, is a type 1 transmembrane homodimeric glycoprotein of 180 kDa [8] and serves as a part of the receptor complex for transforming growth factors beta 1 (TGF-B1) and TGF-B3 [9]. It plays a part in fibrogenesis and angiogenesis [10], and is particularly important for proliferation of endothelial cells and stimulating the activation phase of angiogenesis [11]. It is thus widely expressed in newly-formed vessels versus the old vessels [10, 12], and is a reliable marker for neovascularization in solid tumors, superior to CD31 and CD34 in assessing angiogenesis in growing tumors [13].
In this study, we aimed to undertake a comparative assessment of intratumoral Micro-Vessel Density (MVD) using immunohistochemical expression of CD105 biomarker in PA, MEC, AdCC and normal salivary gland tissue.
2. Materials and Methods
Study specimens
In this cross-sectional comparative study, we selected 20 specimens of PA, 20 specimens of MEC, 20 specimens of AdCC, and 10 specimens of normal salivary gland tissue in paraffin blocks retrieved from the archives of the Pathology Department of Tehran Amir A′lam Hospital, which were collected from 2013 to 2015. All specimen slides, stained with hematoxylin and eosin, were reviewed and rechecked by two independent pathologists to confirm the diagnoses.
Immunohistochemistry
Four-micrometer slices were made by a microtome from each paraffin block and were deparaffinized and rehydrated with xylene and alcohol, respectively. Antigen retrieval was then performed using 1 mM Ethylenediaminetetraacetic Acid (EDTA) buffer with a pH of 8. After irrigation with sterile water and 0.5 M Tris Hydrochloride (Tris-HCL) with a pH of 7, internal peroxidase activity was inhibited by applying 3% oxygen peroxide (H2O2) for 20 minutes.
Slices were then irrigated and incubated in endogenous Avidin/Biotin blocking solution for another 20 minutes. After another irrigation with water and buffer, primary antibody (monoclonal mouse CD105 clone SN6h, 1:100, DAKO, Denmark) was applied for 19 hours at 4°C, according to the manufacturer’s instructions. For reaction amplification, secondary antibodies of biotinylated anti-mouse and Dako streptavidin-biotin-peroxidase were used for 30 minutes each. After meticulous irrigation, staining procedure was performed using 3,3’-diaminobenzidine hydrochloride, and 0.02% H2O2, then Mayer’s hematoxylin solution was used for counterstaining. Negative controls were prepared using the same protocol except for the application of primary antibody, while large B-cell lymphoma and tonsillar tissue were used as positive controls.
Quantitative assessment of MVD
Quantitative assessment of mean MVD was performed according to 2002 global consensus [14] as follows: using low magnification (100x) in multiple slices of each specimen, 10 fields with the highest number of intratumoral vascularization were identified and considered as hotspots. Small vessels in each hotspot were then counted using high magnification (400x). Moreover, any single cell or cluster of cells with a brown color with or without an apparent lumen, branching from an adjacent small vessel, tumoral, or connective tissue was counted as a small vessel. Finally, the average of small vessel counts in these 10 fields was calculated for each sample and considered as the corresponding mean MVD.
Statistical analysis
All statistical analyses were carried out in SPSS version 20 (SPSS Inc., Chicago, IL, USA). The Mann-Whitney U test and the Kruskal-Wallis test were used to compare intratumoral mean MVD between groups. Moreover, between-group differences were evaluated using 1-way ANOVA and Tukey post hoc test. Differences with P values less than 0.05 were considered statistically significant.
3. Results
CD105-expressing vessels were rarely seen in normal salivary tissues. Expression of CD105 was demonstrated in tumors as brown cytoplasmic and membranous staining in endothelial cells of small vessels. Significant differences were observed in intratumoral MVD between normal salivary tissue and AdCC (P<0.017) or MEC (P<0.001), while PA was similar to normal salivary gland tissue in this regard. CD105 expression was significantly higher in the malignant lesions of AdCC and MEC compared to the benign PA (P<0.018 and P<0.001, respectively). In addition, MEC had the highest intratumoral MVD among all lesions, even significantly higher than AdCC (P<0.002). CD105 staining density was also the highest in MEC, while PA showed minimal staining. CD105 expressions and MVD in the three tumoral groups are illustrated in Figures 1-4.
4. Discussion
Diverse clinical course has remained the biggest challenge in management of salivary gland tumors [2], which calls for a comprehensive study of their underlying biology. In general, metastasis is the main factor affecting prognosis and mortality in patients with malignant neoplasms. Some evidence suggests the role of angiogenesis in metastasizing malignant tumors [15]. Higher degree of angiogenesis is associated with poorer prognosis in several cancers, including breast, lung, esophagus, colon, and gastric cancer [7, 8, 12], although evidence to the contrary exists [16-19]. This study aimed to assess neoangiogenesis in the most common benign neoplasm of the salivary glands, PA, as well as important malignant salivary gland tumors namely MEC and AdCC.
Folkman was the first to introduce the concept of angiogenesis [20], and Weidner and colleagues proposed a method to evaluate small vessels in tumoral tissue [2]. Pan-endothelial markers such as CD31, CD34, and Von Willebrand Factor (vWF) have been widely used for this matter in normal and tumoral tissues, but each has its own limitations [21, 22]. CD31 stains similarly positive in small and large vessels, as well as some cells in different carcinomas. CD34, on the other hand, can successfully identify active angiogenic cells, but is not specific and stains positive in mesenchymal cells as well. vWF marker sometimes fails to identify small vessels of normal and tumoral tissues, and may also be expressed in lymphatic vessels [13]. This non-specificity could explain the controversial results in studies on the association of angiogenesis and tumor spread. Introduction of new antibodies, such as CD105, has provided new opportunities for the study of neoangiogenesis in tumors, especially regarding endothelial function and growth [23].
In the present study, MVD was assessed in the intratumoral region because the highest level of angiogenesis due to oxygen and nutrient demands is in this region [2]; however, peri-tumoral vessels more commonly reflect the activities and reactions of the stroma and matrix surrounding the tumor, i.e. the invasiveness of the tumor. CD105 expression was not or minimally observed in normal salivary gland tissues. Moreover, its expression in PA lesions, as the major benign salivary gland neoplasm, was not different than normal tissue. On the other hand, MEC and AdCC demonstrated significantly higher CD105 expressions than PA and normal tissues in our study, along with some other studies [2], which can probably be attributed to their more progressive and aggressive nature. However, this is not always the case, as some malignant tumors have not demonstrated CD105 expression1 probably because of lower oxygen and nutrient needs, resulting in less apparent angiogenesis; this particularly holds true in case of AdCC, which can continue its metabolic activities without high oxygen levels. Nevertheless, evidence of angiogenesis can translate into more aggressive behavior of the tumor [1].
An interesting finding in our study is the significantly higher expression level of CD105 in MEC than in AdCC, which is in accordance with other studies showing the highest angiogenic activity in MEC compared to other benign and malignant salivary gland neoplasms [1, 24]. One can attribute it to the absence of myoepithelial cells in MEC, which are potentially anti-angiogenic [24, 25]. In the absence of proteases and inhibitors of angiogenesis, MEC with its high oxygen demand (due to inability to use glycolysis for its energy source) demonstrates a high MVD index [26].
Sustained hypoxia is toxic to both normal and tumoral cells and usually leads to secretion of angiogenic stimulants such as TGFB by the tumor-related inflammatory cells [27], which in turn upregulates CD105 expression. However, contrary to this theory, tumoral cells might acquire a genetic or epigenetic adaptive advantage in the hypoxic environment, leading to the ability to survive and even proliferate irrespective of the surrounding oxygen level and thus overcoming the need for angiogenesis, which in turn increases their invasion and metastasis potential [28]. Our findings, nevertheless, support the assessment of vascularization and neoangiogenesis as a marker to differentiate malignant from benign neoplasms of the salivary glands.
5. Conclusion
In conclusion, this study showed that CD105 expression and MVD are significantly higher in malignant tumors of the salivary glands than their benign counterparts and normal tissue. This further highlights the role of neoangiogenesis in the pathophysiology of these tumors. We suggest that the association of CD105 expression in malignant salivary gland tumors and disease prognosis be investigated as a future research perspective.
Ethical Considerations
Compliance with ethical guidelines
There is no ethical principle to be considered doing this research.
Funding
This study was funded in part by Babol University of Medical Sciences, and the grant number is 9236710.
Authors contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declare no conflict of interest.
References
- Cardoso SV, Souza KC, Faria PR, Eisenberg AL, Dias FL, Loyola AM. Assessment of angiogenesis by CD105 antigen in epithelial salivary gland neoplasms with diverse metastatic behavior. BMC Cancer. 2009; 9:391. [DOI:10.1186/1471-2407-9-391] [PMID] [PMCID]
- Gleber Netto FO, Florencio TN, de Sousa SF, Abreu MH, Mendonca EF, Aguiar MC. Angiogenesis and lymphangiogenesis in mucoepidermoid carcinoma of minor salivary glands. Journal of Oral Pathology & Medicine . 2010; 41(8):603-9. [DOI:10.1111/j.1600-0714.2012.01153.x] [PMID]
- Neville BW, Damm DD, Allen CM, Chi AC. Oral and maxillofacial pathology. Philadelphia: Saunders; 2015.
- Hillen F, Griffioen AW. Tumour vascularization: sprouting angiogenesis and beyond. Cancer and Metastasis Reviews. 2007; 26(3-4):489-502. [DOI:10.1007/s10555-007-9094-7] [PMID] [PMCID]
- Marioni G, Alessandro ED, Giacomelli L, Staffieri A. CD105 is a marker of tumour vasculature and a potential target for the treatment of head and neck squamous cell carcinoma. Journal of Oral Pathology & Medicine. 2010; 39(5):361-7. [DOI:10.1111/j.1600-0714.2010.00888.x] [PMID]
- Alaeddini M, Salah S, Dehghan F, Eshghyar N, Etemad Moghadam S. Comparison of angiogenesis in keratocystic odontogenic tumours, dentigerous cysts and ameloblastomas. Oral Diseases. 2009; 15(6):422-7. [DOI:10.1111/j.1601-0825.2009.01566.x] [PMID]
- Kumagai Y, Sobajima J, Higashi M, Ishiguro T, Fukuchi M, Ishibashi K. Angiogenesis in superficial esophageal squamous cell carcinoma: Assessment of micro vessel density based on immunostaining for CD34 and CD105. Japanese Journal of Clinical Oncology. 2014; 44(6):526-33. [DOI:10.1093/jjco/hyu039] [PMID]
- Basnaker M, Sr S, Bnvs S. Expression of endoglin (CD105) and microvessel density in oral dysplasia and squamous cell carcinoma. Journal of Clinical and Diagnostic Research for doctors. 2014; 8(9):ZC91-4. [DOI:10.7860/JCDR/2014/9429.4904 ] [PMID] [PMCID]
- Tae K, El Naggar AK, Yoo E, Feng L, Lee JJ, Hong WK, et al. Expression of vascular endothelial growth factor and microvessel density in head and neck tumorigenesis. Clinical Cancer Research. 2000; 6(7):2821-8. [PMID]
- Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: Evidence and potential applications. The FASEB Journal. 2003; 17(9):984-92. [DOI:10.1096/fj.02-0634rev] [PMID]
- Goumans MJ, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch: A balance between two distinct TGF-b receptor signaling pathways. Trends in Cardiovascular Medicine. 2003; 13(7):301-7. [DOI:10.1016/S1050-1738(03)00142-7]
- Fonsatti E, Altomonte M, Nicotra MR, Natali PG, Maio M. Endoglin (CD105): A powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene. 2003; 22(42):6557-63. [DOI:10.1038/sj.onc.1206813] [PMID]
- Eshghyar N, Mohammadi N, Rahrotaban S, Motahhary P, Vahedi Vaez SM. Endoglin (CD105) positive microvessel density and its relationship with lymph node metastasis in squamous cell carcinoma of the tongue. Archives of Iranian Medicine. 2011; 14(4):276-80. [PMID]
- Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. European Journal of Cancer . 2002; 38(12):1564-79. [DOI:10.1016/S0959-8049(02)00094-1]
- Singh S, Sadanandam A, Singh RK. Chemokines in tumor angiogenesis and metastasis. Cancer and Metastasis Reviews. 2007; 26(3-4):453-67. [DOI:10.1007/s10555-007-9068-9] [PMID] [PMCID]
- Lakshman M, Huang X, Ananthanarayanan V, Jovanovic B, Liu Y, Craft CS, et al. Endglin suppresses human prostate cancer metastasis. Clinical & Experimental Metastasis. 2011; 28(1):39-53. [DOI:10.1007/s10585-010-9356-6] [PMID] [PMCID]
- Martone T, Rosso P, Albera R, Migliaretti G, Fraire F, Pignataro L, et al. Prognostic relevance of CD105+ microvessel density in HNSCC patient outcome. Oral Oncology. 2005; 41(2):147-55. [DOI:10.1016/j.oraloncology.2004.08.001] [PMID]
- Schimming R, Marmé D. Endoglin (CD105) expression in squamous cell carcinoma of the oral cavity. Head & Neck. 2002; 24(2):151-6. [DOI:10.1002/hed.10040] [PMID]
- Chien CY, Su CY, Hwang CF, Chuang HC, Hsiao YC, Wu SL, et al. Clinicopathologic signicance of CD105 expression in squamous cell carcinoma of the hypopharynx. Head & Neck. 2006; 28(5):441-6. [DOI:10.1002/hed.20364] [PMID]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000; 407(6801):249-57. [DOI:10.1038/35025220] [PMID]
- Du JR, Jiang Y, Zhang YM, Fu H. Vascular endothelial growth factor and microvascular density in esophageal and gastric carcinomas. World Journal of Gastroenterology. 2003; 9(7):1604-6. [DOI:10.3748/wjg.v9.i7.1604] [PMID] [PMCID]
- Sharma S, Sharma MC, Sarkar C. Morphology of angiogenesis in human cancer: A conceptual overview, histoprognostic perspective and significance of neoangiogenesis. Histopathology. 2005; 46(5):481-9. [DOI:10.1111/j.1365-2559.2005.02142.x] [PMID]
- Cwiklnska A, Sobstyl M, Kwasniewski W, Bednarek W. Microtissue density prognostic factor evaluation based on antigens CD34 and CD 105 in ovarian cancer patients. Annals of Agricultural and Environmental Medicine. 2013; 20(4):838-42. [PMID]
- Costa AF, Demasi AP, Bonfitto VL, Bonfitto JF, Furuse C, Araujo VC. Angiogenesis in salivary carcinomas with and without myoepithelial differentiation. Virchows Archiv. 2008; 453(4):359-67. [DOI:10.1007/s00428-008-0664-z] [PMID]
- Barsky SH, Karlin NJ. Myoepithelial cells: Autocrine and paracrine suppressors of breast cancer progression. Journal of Mammary Gland Biology and Neoplasia. 2005; 10(3):249-60. [DOI:10.1007/s10911-005-9585-5] [PMID]
- Nguyen M, Lee MC, Wang JL, Tomlinson JS, Shao ZM, Alpaugh ML. The human myoepithelial cell displays a multifaceted anti-angiogenic phenotype. Oncogene. 2000; 19(31):3449-59. [DOI:10.1038/sj.onc.1203677] [PMID]
- Gadbail AR, Hande A, Chaudhary M, Nikam A, Gawande M, Patil S, et al. Tumor angiogenesis in keratocystic odontogenic tumor assessed by using CD-105 antigen. Journal of Oral Pathology & Medicine. 2011; 40(3):263-9. [DOI:10.1111/j.1600-0714.2010.00962.x] [PMID]
- Paez Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009; 15(3):220-31. [DOI:10.1016/j.ccr.2009.01.027] [PMID] [PMCID]
Type of Study: Original article |
Subject:
Pathology
Received: 2017/09/28 | Accepted: 2018/01/10 | Published: 2018/03/1
Received: 2017/09/28 | Accepted: 2018/01/10 | Published: 2018/03/1
References
1. Cardoso SV, Souza KC, Faria PR, Eisenberg AL, Dias FL, Loyola AM. Assessment of angiogenesis by CD105 antigen in epithelial salivary gland neoplasms with diverse metastatic behavior. BMC Cancer. 2009; 9:391. [DOI:10.1186/1471-2407-9-391] [PMID] [PMCID] [DOI:10.1186/1471-2407-9-391]
2. Gleber Netto FO, Florencio TN, de Sousa SF, Abreu MH, Mendonca EF, Aguiar MC. Angiogenesis and lymphangiogenesis in mucoepidermoid carcinoma of minor salivary glands. Journal of Oral Pathology & Medicine . 2010; 41(8):603-9. [DOI:10.1111/j.1600-0714.2012.01153.x] [PMID] [DOI:10.1111/j.1600-0714.2012.01153.x]
3. Neville BW, Damm DD, Allen CM, Chi AC. Oral and maxillofacial pathology. Philadelphia: Saunders; 2015.
4. Hillen F, Griffioen AW. Tumour vascularization: sprouting angiogenesis and beyond. Cancer and Metastasis Reviews. 2007; 26(3-4):489-502. [DOI:10.1007/s10555-007-9094-7] [PMID] [PMCID] [DOI:10.1007/s10555-007-9094-7]
5. Marioni G, Alessandro ED, Giacomelli L, Staffieri A. CD105 is a marker of tumour vasculature and a potential target for the treatment of head and neck squamous cell carcinoma. Journal of Oral Pathology & Medicine. 2010; 39(5):361-7. [DOI:10.1111/j.1600-0714.2010.00888.x] [PMID] [DOI:10.1111/j.1600-0714.2010.00888.x]
6. Alaeddini M, Salah S, Dehghan F, Eshghyar N, Etemad Moghadam S. Comparison of angiogenesis in keratocystic odontogenic tumours, dentigerous cysts and ameloblastomas. Oral Diseases. 2009; 15(6):422-7. [DOI:10.1111/j.1601-0825.2009.01566.x] [PMID] [DOI:10.1111/j.1601-0825.2009.01566.x]
7. Kumagai Y, Sobajima J, Higashi M, Ishiguro T, Fukuchi M, Ishibashi K. Angiogenesis in superficial esophageal squamous cell carcinoma: Assessment of micro vessel density based on immunostaining for CD34 and CD105. Japanese Journal of Clinical Oncology. 2014; 44(6):526-33. [DOI:10.1093/jjco/hyu039] [PMID] [DOI:10.1093/jjco/hyu039]
8. Basnaker M, Sr S, Bnvs S. Expression of endoglin (CD105) and microvessel density in oral dysplasia and squamous cell carcinoma. Journal of Clinical and Diagnostic Research for doctors. 2014; 8(9):ZC91-4. [DOI:10.7860/JCDR/2014/9429.4904 ] [PMID] [PMCID] [DOI:10.7860/JCDR/2014/9429.4904]
9. Tae K, El Naggar AK, Yoo E, Feng L, Lee JJ, Hong WK, et al. Expression of vascular endothelial growth factor and microvessel density in head and neck tumorigenesis. Clinical Cancer Research. 2000; 6(7):2821-8. [PMID] [PMID]
10. Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: Evidence and potential applications. The FASEB Journal. 2003; 17(9):984-92. [DOI:10.1096/fj.02-0634rev] [PMID] [DOI:10.1096/fj.02-0634rev]
11. Goumans MJ, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch: A balance between two distinct TGF-b receptor signaling pathways. Trends in Cardiovascular Medicine. 2003; 13(7):301-7. [DOI:10.1016/S1050-1738(03)00142-7] [DOI:10.1016/S1050-1738(03)00142-7]
12. Fonsatti E, Altomonte M, Nicotra MR, Natali PG, Maio M. Endoglin (CD105): A powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene. 2003; 22(42):6557-63. [DOI:10.1038/sj.onc.1206813] [PMID] [DOI:10.1038/sj.onc.1206813]
13. Eshghyar N, Mohammadi N, Rahrotaban S, Motahhary P, Vahedi Vaez SM. Endoglin (CD105) positive microvessel density and its relationship with lymph node metastasis in squamous cell carcinoma of the tongue. Archives of Iranian Medicine. 2011; 14(4):276-80. [PMID] [PMID]
14. Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. European Journal of Cancer . 2002; 38(12):1564-79. [DOI:10.1016/S0959-8049(02)00094-1] [DOI:10.1016/S0959-8049(02)00094-1]
15. Singh S, Sadanandam A, Singh RK. Chemokines in tumor angiogenesis and metastasis. Cancer and Metastasis Reviews. 2007; 26(3-4):453-67. [DOI:10.1007/s10555-007-9068-9] [PMID] [PMCID] [DOI:10.1007/s10555-007-9068-9]
16. Lakshman M, Huang X, Ananthanarayanan V, Jovanovic B, Liu Y, Craft CS, et al. Endglin suppresses human prostate cancer metastasis. Clinical & Experimental Metastasis. 2011; 28(1):39-53. [DOI:10.1007/s10585-010-9356-6] [PMID] [PMCID] [DOI:10.1007/s10585-010-9356-6]
17. Martone T, Rosso P, Albera R, Migliaretti G, Fraire F, Pignataro L, et al. Prognostic relevance of CD105+ microvessel density in HNSCC patient outcome. Oral Oncology. 2005; 41(2):147-55. [DOI:10.1016/j.oraloncology.2004.08.001] [PMID] [DOI:10.1016/j.oraloncology.2004.08.001]
18. Schimming R, Marmé D. Endoglin (CD105) expression in squamous cell carcinoma of the oral cavity. Head & Neck. 2002; 24(2):151-6. [DOI:10.1002/hed.10040] [PMID] [DOI:10.1002/hed.10040]
19. Chien CY, Su CY, Hwang CF, Chuang HC, Hsiao YC, Wu SL, et al. Clinicopathologic signicance of CD105 expression in squamous cell carcinoma of the hypopharynx. Head & Neck. 2006; 28(5):441-6. [DOI:10.1002/hed.20364] [PMID] [DOI:10.1002/hed.20364]
20. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000; 407(6801):249-57. [DOI:10.1038/35025220] [PMID] [DOI:10.1038/35025220]
21. Du JR, Jiang Y, Zhang YM, Fu H. Vascular endothelial growth factor and microvascular density in esophageal and gastric carcinomas. World Journal of Gastroenterology. 2003; 9(7):1604-6. [DOI:10.3748/wjg.v9.i7.1604] [PMID] [PMCID] [DOI:10.3748/wjg.v9.i7.1604]
22. Sharma S, Sharma MC, Sarkar C. Morphology of angiogenesis in human cancer: A conceptual overview, histoprognostic perspective and significance of neoangiogenesis. Histopathology. 2005; 46(5):481-9. [DOI:10.1111/j.1365-2559.2005.02142.x] [PMID] [DOI:10.1111/j.1365-2559.2005.02142.x]
23. Cwiklnska A, Sobstyl M, Kwasniewski W, Bednarek W. Microtissue density prognostic factor evaluation based on antigens CD34 and CD 105 in ovarian cancer patients. Annals of Agricultural and Environmental Medicine. 2013; 20(4):838-42. [PMID] [PMID]
24. Costa AF, Demasi AP, Bonfitto VL, Bonfitto JF, Furuse C, Araujo VC. Angiogenesis in salivary carcinomas with and without myoepithelial differentiation. Virchows Archiv. 2008; 453(4):359-67. [DOI:10.1007/s00428-008-0664-z] [PMID] [DOI:10.1007/s00428-008-0664-z]
25. Barsky SH, Karlin NJ. Myoepithelial cells: Autocrine and paracrine suppressors of breast cancer progression. Journal of Mammary Gland Biology and Neoplasia. 2005; 10(3):249-60. [DOI:10.1007/s10911-005-9585-5] [PMID] [DOI:10.1007/s10911-005-9585-5]
26. Nguyen M, Lee MC, Wang JL, Tomlinson JS, Shao ZM, Alpaugh ML. The human myoepithelial cell displays a multifaceted anti-angiogenic phenotype. Oncogene. 2000; 19(31):3449-59. [DOI:10.1038/sj.onc.1203677] [PMID] [DOI:10.1038/sj.onc.1203677]
27. Gadbail AR, Hande A, Chaudhary M, Nikam A, Gawande M, Patil S, et al. Tumor angiogenesis in keratocystic odontogenic tumor assessed by using CD-105 antigen. Journal of Oral Pathology & Medicine. 2011; 40(3):263-9. [DOI:10.1111/j.1600-0714.2010.00962.x] [PMID] [DOI:10.1111/j.1600-0714.2010.00962.x]
28. Paez Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009; 15(3):220-31. [DOI:10.1016/j.ccr.2009.01.027] [PMID] [PMCID] [DOI:10.1016/j.ccr.2009.01.027]
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |