Fri, Apr 26, 2024
Volume 7, Issue 3 (9-2018)
2018, 7(3): 103-108 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ashraf H, Mojtahed Bidabadi M, Noghlachi T, Darmiani S. Controlling Postendodontic Pain in Comparison to Placebo: A Randomized Double-blind Clinical Trial. Journal title 2018; 7 (3) :103-108
URL: http://3dj.gums.ac.ir/article-1-320-en.html
URL: http://3dj.gums.ac.ir/article-1-320-en.html
1- Professor, Department of Endodontics, Shool of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Endodontist.
3- DDS, Dentist.
4- Assistant professor, Department of Endodontics, Shool of Dentistry, Birjand University of Medical Sciences, Birjand, Iran. , soheiladarmiani@yahoo.com
2- Endodontist.
3- DDS, Dentist.
4- Assistant professor, Department of Endodontics, Shool of Dentistry, Birjand University of Medical Sciences, Birjand, Iran. , soheiladarmiani@yahoo.com
Full-Text [PDF 681 kb]
(696 Downloads)
| Abstract (HTML) (2512 Views)
Full-Text: (633 Views)
1. Introduction
revious studies have shown that Root Canal Treatments (RCTs) cause more continuous and severe postoperative pain than other dental operations [1, 2]. Postoperative pain following RCTs has been reported to occur in 25-40% of all endodontic patients [3-5]. A significant relationship exists between pre-endodontic and post-endodontic pain. Patients with severe preoperative pain tend to have more severe operative and postoperative pain than patients with mild or no preoperative pain. Biological, chemical, and mechanical damages to periapical tissues during endodontic treatment cause an acute inflammation within the periradicular tissues [6, 7]. Certain inflammatory mediators (prostaglandins [PGs]), leukotrienes, bradykinin, etc.) have been associated with this inflammatory process [4]. PGs elevate vascular permeability, increase chemotactic activity, induce fever, and increase the sensitivity of pain receptors to other active inflammatory mediators [3, 4].
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) inhibit PG synthesis by decreasing the activity of the cyclooxygenase (COX) enzyme. Researchers discovered that the COX enzyme exists as two separate entities, cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). COX-1 synthesizes protective PGs, which preserve the integrity of the stomach lining and maintain normal renal function in a compromised kidney. COX-2 is induced by proinflammatory cytokines and growth of the factors, which implies that COX-2 plays a role in both inflammation and control of cell growth. The discovery of COX-2 has made it possible for researchers to design drugs that reduce inflammation without removing the protective PGs of the stomach and kidney [3, 4].
A variety of approaches have been suggested for the management of interappointment pain. These approaches include occlusion reduction, prescription of analgesics; also, the use of steroidal and inflammatory response should be considered for the prevention and control of post-endodontic pain. Therefore, systematic drugs have been used to reduce the severity of post-treatment pain. However, a definitive anti-inflammatory protocol to prevent and control the occurrence of post-endodontic pain has not yet been established [5-7].
In comparison to repeated doses during the postoperative period, a preoperative single oral dose of NSAIDs can modulate the release of inflammatory mediators and reduce the occurrence of side effects. The maximum benefit of the anti-inflammatory is obtained when therapeutic levels are achieved before tissue manipulation.
Celecoxib is the NSAIDs that have been proved to be an effective analgesic for osteoarthritis, rheumatoid arthritis, juvenile rheumatoid arthritis, ankylosis spondylitis, primary dysmenorrhea, and acute pain in adults [2, 5]. Celecoxib has been proved to have a high affinity to block the COX-2 enzyme [8]. Celecoxib (brand names of Celebrex, Celebra, and Onsenal) was one of the first of the new generation of NSAIDs known as selective COX-2 inhibitors or “coxibs” and Celebrex® is currently licensed for the relief of osteoarthritis and rheumatoid arthritis pain in many countries around the world including the United Kingdom and the United States of America.
It is available by prescription only in many countries in 50 mg, 100 mg, 200 mg, or 400 mg capsules, but the generic formulation is available in some parts of Asia and the Far East where patents have expired. In acute painful conditions, such as postoperative pain, sometimes up to 400 mg as a single or starting dose is prescribed [9]. No clinical trials have been carried in dentistry to compare celecoxib and Celebrex. The purpose of this study was to clinically evaluate the effectiveness of either prophylactic celecoxib (Shimidaroo) or Celebrex (Pfizer) in reducing postendodontic pain compared with placebo.
2. Materials and Methods
Informed consent was obtained from all the subjects of this study approved by the Ethics Committee of the Dental School of Shahid Beheshti University, Tehran, Iran. Random simple sampling method was performed by balanced (permuted) block randomization. The subjects were selected among the patients referred to the Endodontic Department of Shahid Beheshti Dental School based on the following inclusion criteria: the tooth was multirooted with vital pulp based on clinical examinations and vitality tests, and the patients should have moderate to severe pain based on thermal reactions. Exclusion criteria were taking any analgesia within 6h before intervention, acute endodontic or periodontal abscess, pregnancy and lactation, mental disabilities, systemic disease, and adverse reaction or allergy to celecoxib and Celebrex.
Drugs were purchased from Shimidaroo-Pfizer. Each patient was anesthetized with a solution of 2% lidocaine plus 1:80000 epinephrine (Daroupakhsh Co, Tehran, Iran) followed by rubber dam isolation, access cavity preparation, and cleaning and shaping the canals. The RCT procedure was conducted using a passive step-back technique. The apical region was prepared to size 35 K-file (Maileffer, Dentsply, Ballaigues, Switzerland) and the canal was irrigated with 2.5% sodium hypochlorite. When instrumentation was completed, the canals were rinsed thoroughly and dried with paper points. They were filled with gutta-percha and AH 26 sealer (Dentsply-Germany) using lateral condensation technique. A cotton pellet was placed in the access cavity, which was restored with cavit (3M ESPE, St. Paul, MN, USA).
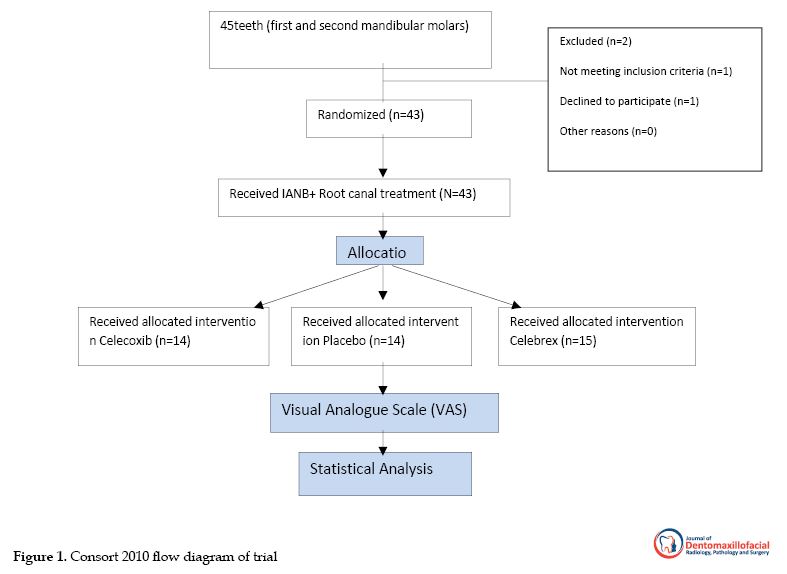
To maintain the double-blind design, a second investigator provided the three agents with the capsule so that the patients were not aware of the medication they were taking. Immediately the pain perception of the patients was recorded after the dental intervention, and they were instructed to complete a pain chart 4, 8, 12, 24, and 48 h after initiation of the dental intervention. They were also told to take the extra medication and record it in the chart (500 mg acetaminophen) only if they needed it. The method used to measure clinical pain intensity was the Visual Analogue Scale (VAS), which consisted of a 170 mm anchor line by two extremes: no pain and very severe pain. Then they were asked to put a mark on the line that represented their level of perceived pain. Consequently, the pain intensity was categorized in four levels: none (0), mild (1-54), moderate (54-144), and severe (144-170). For statistical analysis, we used SPSS V. 16 (SPSS Inc. Chicago, USA). The data were analyzed by the Mann-Whitney and Freidman tests. Significance was set at 0.05 (Figure 1).
revious studies have shown that Root Canal Treatments (RCTs) cause more continuous and severe postoperative pain than other dental operations [1, 2]. Postoperative pain following RCTs has been reported to occur in 25-40% of all endodontic patients [3-5]. A significant relationship exists between pre-endodontic and post-endodontic pain. Patients with severe preoperative pain tend to have more severe operative and postoperative pain than patients with mild or no preoperative pain. Biological, chemical, and mechanical damages to periapical tissues during endodontic treatment cause an acute inflammation within the periradicular tissues [6, 7]. Certain inflammatory mediators (prostaglandins [PGs]), leukotrienes, bradykinin, etc.) have been associated with this inflammatory process [4]. PGs elevate vascular permeability, increase chemotactic activity, induce fever, and increase the sensitivity of pain receptors to other active inflammatory mediators [3, 4].
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) inhibit PG synthesis by decreasing the activity of the cyclooxygenase (COX) enzyme. Researchers discovered that the COX enzyme exists as two separate entities, cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). COX-1 synthesizes protective PGs, which preserve the integrity of the stomach lining and maintain normal renal function in a compromised kidney. COX-2 is induced by proinflammatory cytokines and growth of the factors, which implies that COX-2 plays a role in both inflammation and control of cell growth. The discovery of COX-2 has made it possible for researchers to design drugs that reduce inflammation without removing the protective PGs of the stomach and kidney [3, 4].
A variety of approaches have been suggested for the management of interappointment pain. These approaches include occlusion reduction, prescription of analgesics; also, the use of steroidal and inflammatory response should be considered for the prevention and control of post-endodontic pain. Therefore, systematic drugs have been used to reduce the severity of post-treatment pain. However, a definitive anti-inflammatory protocol to prevent and control the occurrence of post-endodontic pain has not yet been established [5-7].
In comparison to repeated doses during the postoperative period, a preoperative single oral dose of NSAIDs can modulate the release of inflammatory mediators and reduce the occurrence of side effects. The maximum benefit of the anti-inflammatory is obtained when therapeutic levels are achieved before tissue manipulation.
Celecoxib is the NSAIDs that have been proved to be an effective analgesic for osteoarthritis, rheumatoid arthritis, juvenile rheumatoid arthritis, ankylosis spondylitis, primary dysmenorrhea, and acute pain in adults [2, 5]. Celecoxib has been proved to have a high affinity to block the COX-2 enzyme [8]. Celecoxib (brand names of Celebrex, Celebra, and Onsenal) was one of the first of the new generation of NSAIDs known as selective COX-2 inhibitors or “coxibs” and Celebrex® is currently licensed for the relief of osteoarthritis and rheumatoid arthritis pain in many countries around the world including the United Kingdom and the United States of America.
It is available by prescription only in many countries in 50 mg, 100 mg, 200 mg, or 400 mg capsules, but the generic formulation is available in some parts of Asia and the Far East where patents have expired. In acute painful conditions, such as postoperative pain, sometimes up to 400 mg as a single or starting dose is prescribed [9]. No clinical trials have been carried in dentistry to compare celecoxib and Celebrex. The purpose of this study was to clinically evaluate the effectiveness of either prophylactic celecoxib (Shimidaroo) or Celebrex (Pfizer) in reducing postendodontic pain compared with placebo.
2. Materials and Methods
Informed consent was obtained from all the subjects of this study approved by the Ethics Committee of the Dental School of Shahid Beheshti University, Tehran, Iran. Random simple sampling method was performed by balanced (permuted) block randomization. The subjects were selected among the patients referred to the Endodontic Department of Shahid Beheshti Dental School based on the following inclusion criteria: the tooth was multirooted with vital pulp based on clinical examinations and vitality tests, and the patients should have moderate to severe pain based on thermal reactions. Exclusion criteria were taking any analgesia within 6h before intervention, acute endodontic or periodontal abscess, pregnancy and lactation, mental disabilities, systemic disease, and adverse reaction or allergy to celecoxib and Celebrex.
Drugs were purchased from Shimidaroo-Pfizer. Each patient was anesthetized with a solution of 2% lidocaine plus 1:80000 epinephrine (Daroupakhsh Co, Tehran, Iran) followed by rubber dam isolation, access cavity preparation, and cleaning and shaping the canals. The RCT procedure was conducted using a passive step-back technique. The apical region was prepared to size 35 K-file (Maileffer, Dentsply, Ballaigues, Switzerland) and the canal was irrigated with 2.5% sodium hypochlorite. When instrumentation was completed, the canals were rinsed thoroughly and dried with paper points. They were filled with gutta-percha and AH 26 sealer (Dentsply-Germany) using lateral condensation technique. A cotton pellet was placed in the access cavity, which was restored with cavit (3M ESPE, St. Paul, MN, USA).
To maintain the double-blind design, a second investigator provided the three agents with the capsule so that the patients were not aware of the medication they were taking. Immediately the pain perception of the patients was recorded after the dental intervention, and they were instructed to complete a pain chart 4, 8, 12, 24, and 48 h after initiation of the dental intervention. They were also told to take the extra medication and record it in the chart (500 mg acetaminophen) only if they needed it. The method used to measure clinical pain intensity was the Visual Analogue Scale (VAS), which consisted of a 170 mm anchor line by two extremes: no pain and very severe pain. Then they were asked to put a mark on the line that represented their level of perceived pain. Consequently, the pain intensity was categorized in four levels: none (0), mild (1-54), moderate (54-144), and severe (144-170). For statistical analysis, we used SPSS V. 16 (SPSS Inc. Chicago, USA). The data were analyzed by the Mann-Whitney and Freidman tests. Significance was set at 0.05 (Figure 1).
3. Results
Pain scores from 45 subjects were recorded by a 170-mm VAS. Two subjects were excluded from the study, and only 43 subjects were analyzed. Fifteen subjects received Celebrex, 14 received celecoxib, and 14 received a placebo before dental intervention. According to Table 1, there were no statistically significant differences among study groups considering age, gender, teeth, and diagnosis.
Pain scores from 45 subjects were recorded by a 170-mm VAS. Two subjects were excluded from the study, and only 43 subjects were analyzed. Fifteen subjects received Celebrex, 14 received celecoxib, and 14 received a placebo before dental intervention. According to Table 1, there were no statistically significant differences among study groups considering age, gender, teeth, and diagnosis.
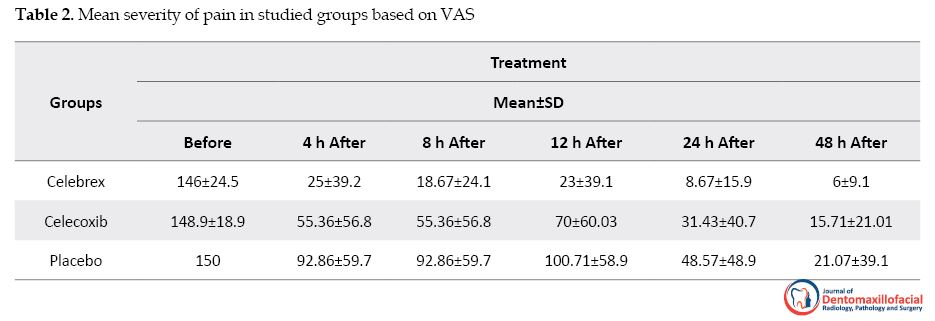
Table 2 presents the mean pain scores at various time intervals in different study groups. At the 4 (P=0.04), 8 (P=0.01), 12 (P=0.03), 24 (P=0.04), and 48 h (P=0.02) periods, Celebrex provided significantly better pain relief than placebo. There was no significant difference (P=0.065) between celecoxib and placebo during 48 h after initiation of dental intervention. However, when comparing Celebrex to celecoxib, only at 12h period, Celebrex was significantly more effective at reducing pain (P=0.03). No side effects were reported for any of the medications used.
4. Discussion
In this study, we investigated the effect of prophylactic Celebrex and celecoxib on controlling postendodontic pain. Prophylactic oral administration of NSAIDs has been proved to reduce postoperative pain in RCT models. Preemptive administration of NSAIDs before conventional RCT can block the COX pathway and block the pain sensation before its start [9].
Shirvani et al. in a systematic review and meta-analysis concluded that administration of NSAIDs could manage postoperative endodontic pain [10]. In this study, NSAIDs were administered 30 minutes before conventional RCT. A single oral dose of the drug prescribed 30 minutes before endodontic procedures might be appropriate when the endodontic instruments and irrigating solutions reach the periapical region. The drug will have achieved therapeutic levels in the tissues.
Protocols for measuring pain following endodontic treatments differ from oral surgeries in several respects, because evaluations of anti-inflammatory and analgesic drugs in oral surgical procedures cannot be directly extrapolated for determining the appropriate approach to treat endodontic pain [5]. We measured post-treatment pain level using a standard VAS scale at pre-defined time tables of 4, 8, 12, and 24 h after treatment. VAS is a valid and reliable method; it is easy to understand, reproducible, and widely used in the endodontic literature [11-13]. So it was applied to measure pain in the present study. The failure rate of VAS is between 4% and 11%, but this can be reduced if the tool is carefully explained to the patient.
This double-blind, randomized study minimized bias and allowed sufficient comparison between the groups. The samples were similarly distributed regarding their age, gender, and teeth (first and second mandibular molars). Patients with moderate to severe pain in preoperative pain have been selected as the primary determinant of postoperative pain or flare-up [13, 14]. Nonetheless, the mean pain intensity (VAS) was similar in the pretreatment for all groups. Although control of the variables will need to limit bias and provide reliable results, future clinical trials are still required.
Patients of the placebo group reported increased pain during 8-12 h after the procedure, which indicates the need for additional (escape) medication. In this study, placebo group showed 42.8% pain reduction from the VAS baseline pain compared to the VAS measurements recorded in the 24-48 h following treatment. Menhinick et al. also in a randomized, double-blind study showed a mean pain reduction of 71% in the placebo group [15]. In the present research, Heft-Parker VAS was applied to estimate the patient’s pain, before and after the endodontic treatment. Most of the previous studies had also used this scale to analyze the pain perception of the patients [4, 16-18].
Endodontic literature is full of studies, which compared the effectiveness of one, two, or more different drugs with different mechanisms with placebo in reducing the pain. The results of Cheung et al. study show that the onset and magnitude of pain relief with 400mg celecoxib and 400mg ibuprofen are comparable. In addition, patients who received celecoxib as a single dose had a significantly longer time to use of rescue medication and had higher pain relief scores later in the study than those who received ibuprofen [19]. Mirzaie et al. found that the use of celecoxib before treatment reduces postendodontic pain [20]. Our study is the first research that evaluates the effect of Celebrex. We found that administering of Celebrex before RCT was more effective at reducing postendodontic pain at all times after initiation of treatment compared with Celecoxib and placebo effects.
Although among the studied populations there was no significant side effect mentioned by the subjects, long-term use of these drugs may create some common side effects such as nausea, dizziness, headache, gastrointestinal discomfort, etc. However, it is recommended that long-term studies be conducted in the future to obtain more valid results.
5. Conclusion
Regarding the results of this double-blind, randomized clinical trial study, the drug regimen of 400 mg of Celebrex before endodontic treatment is effective for post-treatment pain relief in molar teeth with irreversible pulpitis.
In summary, postendodontic pain treatment by Celebrex is effective. The Celebrex may serve as a preventive analgesic drug. It has long-term efficacy, low toxicity for the GI tract, and no inhibition of platelet function. So it is useful in postendodontic pain, especially in patients with GI diseases. Further clinical studies, examining different clinical conditions and other regimens for Celebrex treatment should be carried out to elucidate the potential of this drug in the context of endodontic therapy.
Ethical Considerations
Compliance with ethical guidelines
All procedures performed in this study were in accordance with the ethical standards of the institutional and or national research committee and compatible with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
The present paper was extracted from the PhD. thesis of Tannaz Noghlachi in School of Dentistry, Shahid Beheshti University of Medical Sciences.
Authors contribution's
All authors contributed in preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
In this study, we investigated the effect of prophylactic Celebrex and celecoxib on controlling postendodontic pain. Prophylactic oral administration of NSAIDs has been proved to reduce postoperative pain in RCT models. Preemptive administration of NSAIDs before conventional RCT can block the COX pathway and block the pain sensation before its start [9].
Shirvani et al. in a systematic review and meta-analysis concluded that administration of NSAIDs could manage postoperative endodontic pain [10]. In this study, NSAIDs were administered 30 minutes before conventional RCT. A single oral dose of the drug prescribed 30 minutes before endodontic procedures might be appropriate when the endodontic instruments and irrigating solutions reach the periapical region. The drug will have achieved therapeutic levels in the tissues.
Protocols for measuring pain following endodontic treatments differ from oral surgeries in several respects, because evaluations of anti-inflammatory and analgesic drugs in oral surgical procedures cannot be directly extrapolated for determining the appropriate approach to treat endodontic pain [5]. We measured post-treatment pain level using a standard VAS scale at pre-defined time tables of 4, 8, 12, and 24 h after treatment. VAS is a valid and reliable method; it is easy to understand, reproducible, and widely used in the endodontic literature [11-13]. So it was applied to measure pain in the present study. The failure rate of VAS is between 4% and 11%, but this can be reduced if the tool is carefully explained to the patient.
This double-blind, randomized study minimized bias and allowed sufficient comparison between the groups. The samples were similarly distributed regarding their age, gender, and teeth (first and second mandibular molars). Patients with moderate to severe pain in preoperative pain have been selected as the primary determinant of postoperative pain or flare-up [13, 14]. Nonetheless, the mean pain intensity (VAS) was similar in the pretreatment for all groups. Although control of the variables will need to limit bias and provide reliable results, future clinical trials are still required.
Patients of the placebo group reported increased pain during 8-12 h after the procedure, which indicates the need for additional (escape) medication. In this study, placebo group showed 42.8% pain reduction from the VAS baseline pain compared to the VAS measurements recorded in the 24-48 h following treatment. Menhinick et al. also in a randomized, double-blind study showed a mean pain reduction of 71% in the placebo group [15]. In the present research, Heft-Parker VAS was applied to estimate the patient’s pain, before and after the endodontic treatment. Most of the previous studies had also used this scale to analyze the pain perception of the patients [4, 16-18].
Endodontic literature is full of studies, which compared the effectiveness of one, two, or more different drugs with different mechanisms with placebo in reducing the pain. The results of Cheung et al. study show that the onset and magnitude of pain relief with 400mg celecoxib and 400mg ibuprofen are comparable. In addition, patients who received celecoxib as a single dose had a significantly longer time to use of rescue medication and had higher pain relief scores later in the study than those who received ibuprofen [19]. Mirzaie et al. found that the use of celecoxib before treatment reduces postendodontic pain [20]. Our study is the first research that evaluates the effect of Celebrex. We found that administering of Celebrex before RCT was more effective at reducing postendodontic pain at all times after initiation of treatment compared with Celecoxib and placebo effects.
Although among the studied populations there was no significant side effect mentioned by the subjects, long-term use of these drugs may create some common side effects such as nausea, dizziness, headache, gastrointestinal discomfort, etc. However, it is recommended that long-term studies be conducted in the future to obtain more valid results.
5. Conclusion
Regarding the results of this double-blind, randomized clinical trial study, the drug regimen of 400 mg of Celebrex before endodontic treatment is effective for post-treatment pain relief in molar teeth with irreversible pulpitis.
In summary, postendodontic pain treatment by Celebrex is effective. The Celebrex may serve as a preventive analgesic drug. It has long-term efficacy, low toxicity for the GI tract, and no inhibition of platelet function. So it is useful in postendodontic pain, especially in patients with GI diseases. Further clinical studies, examining different clinical conditions and other regimens for Celebrex treatment should be carried out to elucidate the potential of this drug in the context of endodontic therapy.
Ethical Considerations
Compliance with ethical guidelines
All procedures performed in this study were in accordance with the ethical standards of the institutional and or national research committee and compatible with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
The present paper was extracted from the PhD. thesis of Tannaz Noghlachi in School of Dentistry, Shahid Beheshti University of Medical Sciences.
Authors contribution's
All authors contributed in preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
- Gaskell H, Derry S, Wiffen PJ, Moore RA. Single dose oral ketoprofen or dexketoprofen for acute postoperative pain in adults. Cochrane Database of Systematic Reviews. 2017; 2017(5):CD007355. [DOI:10.1002/14651858.CD007355.pub3] [PMID] [PMCID]
- Ashley PF, Parekh S, Moles DR, Anand P, Behbehani A. Preoperative analgesics for additional pain relief in children and adolescents having dental treatment. Cochrane Database of Systematic Reviews. 2016; 8:CD008392. [DOI:10.1002/14651858.CD008392.pub2] [PMID]
- Moore PA, Ziegler KM, Lipman RD, Aminoshariae A, Carrasco-Labra A, Mariotti A. Benefits and harms associated with analgesic medications used in the management of acute dental pain: An overview of systematic reviews. Journal of the American Dental Association. 2018; 149(4):256-65. [DOI:10.1016/j.adaj.2018.02.012] [PMID]
- Jalalzadeh SM, Mamavi A, Shahriari S, Santos FA, Pochapski MT. Effect of pretreatment prednisolone on postendodontic pain: A double-blind parallel-randomized clinical trial. Journal of Endodontics. 2010; 36(6):978-81. [DOI:10.1016/j.joen.2010.03.015] [PMID]
- Taneja P, Pattni A, Pearson D. What’s new in the management of post-operative pain in dentistry. SAAD Digest. 2015; 31:3-7. [PMID]
- Su Y, Wang C, Ye L. Healing rate and post-obturation pain of single-versus multiple-visit endodontic treatment for infected root canals: A systematic review. Journal of Endodontics. 2011; 37(2):125-32. [DOI:10.1016/j.joen.2010.09.005] [PMID]
- Pak JG, White SN. Pain prevalence and severity before, during, and after root canal treatment: A systematic review. Journal of Endodontics. 2011; 37(4):429-38. [DOI:10.1016/j.joen.2010.12.016] [PMID]
- Chen LC, Elliott RA, Ashcroft DM. Systematic review of the analgesic efficacy and tolerability of COX‐2 inhibitors in post‐operative pain control. Journal of Clinical Pharmacy and Therapeutics. 2004; 29(3):215-29. [DOI:10.1111/j.1365-2710.2004.00558.x] [PMID]
- Straube S, Derry S, McQuay HJ, Moore RA. Effect of preoperative Cox‐II‐selective NSAIDs (coxibs) on postoperative outcomes: A systematic review of randomized studies. Acta anaesthesiologica Scandinavica. 2005; 49(5):601-13. [DOI:10.1111/j.1399-6576.2005.00666.x] [PMID]
- Shirvani a, Shamszadeh S, Eghbal M.J, Asgary S. The efficacy of non‐steroidal anti‐inflammatory drugs and/or paracetamol on post‐operative endodontic pain. Journal of Oral Rehabilitation. 2017; 44(9):709-21. [DOI:10.1111/joor.12519] [PM ID]
- Alí A, Olivieri JG, Duran-Sindreu F, Abella F, Roig M, García-Font M. Influence of preoperative pain intensity on postoperative pain after root canal treatment: A prospective clinical study. Journal of Dentistry. 2016; 45:39-42. [DOI:10.1016/j.jdent.2015.12.002] [PMID]
- Ryan JL, Jureidini B, Hodges JS, Baisden M, Swift JQ, Bowles WR. Gender differences in analgesia for endodontic pain. Journal of Endodontics. 2008; 34(5):552-6. [DOI:10.1016/j.joen.2008.01.021] [PMID]
- Sheikh A, Agwan MA, Amin M, Khan MA, Sheikh I, Shah SI. Comparison of ibuprofen and celecoxib for controlling postendodontic pain. Journal of the Pakistan Dental Association. 2014; 23(3):106-11.
- Nagendrababu V, Gutmann JL. Factors associated with postobturation pain following single-visit nonsurgical root canal treatment: A systematic review. Quintessence International. 2017; 48(3) 193-208. [DOI:10.3290/j.qi.a36894] [PMID]
- Menhinick KA, Gutmann JL, Regan JD, Taylor SE, Buschang PH. The efficacy of pain control following nonsurgical root canal treatment using ibuprofen or a combination of ibuprofen and acetaminophen in a randomized, double‐blind, placebo‐controlled study. International Endodontic Journal. 2004; 37(8):531-41. [DOI:10.1111/j.1365-2591.2004.00836.x] [PMID]
- Polycarpou N, Ng YL, Canavan D, Moles DR, Gulabivala K. Prevalence of persistent pain after endodontic treatment and factors affecting its occurrence in cases with complete radiographichealing. International Endodontic Journal. 2005; 38(3):169-78. [DOI:10.1111/j.1365-2591.2004.00923.x] [PMID]
- Arslan H, Topcuoglu HS, Aladag H. Effectiveness of tenoxicam and ibuprofen for pain prevention following endodontic therapy in comparison to placebo: A randomized double-blind clinical trial. Journal of Oral Science. 2011; 53(2):157-61. [DOI:10.2334/josnusd.53.157] [PMID]
- Terry R, Posadzki P, Watson LK, Ernst E. The use of ginger (Zingiber officinale) for the treatment of pain: A systematic review of clinical trials. Pain Medicine. 2011; 12(12):1808-18. [DOI:10.1111/j.1526-4637.2011.01261.x] [PMID]
- Cheung R, Krishnaswami S, Kowalski K. Analgesic efficacy of celecoxib in postoperative oral surgery pain: A single-dose, two-center, randomized, double-blind, active-and placebo-controlled study. Clinical therapeutics. 2007; 29(11):2498-510. [DOI:10.1016/j.clinthera.2007.12.008] [PMID]
- Mirzaie M, Kavosi A, Atbaie A, Moazami F, Nooribaiat Sh. [Effect of premedication with Celecoxib and Gelofen on reduction of post-endodontic pain (Persian)]. Journal of Dental Medicine. 2011; 24(3):172-80.
Type of Study: Original article |
Subject:
So on
Received: 2018/02/3 | Accepted: 2018/07/10 | Published: 2018/09/1
Received: 2018/02/3 | Accepted: 2018/07/10 | Published: 2018/09/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |