Wed, Apr 24, 2024
Volume 7, Issue 4 (12-2018)
2018, 7(4): 151-156 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ahmadian Babaki F, Daneshvar S H, Moslemi M, Zare M. Effect of Different Fluoridated Toothpastes on the Enamel Microhardness of Primary Teeth. Journal title 2018; 7 (4) :151-156
URL: http://3dj.gums.ac.ir/article-1-329-en.html
URL: http://3dj.gums.ac.ir/article-1-329-en.html
1- Assistant Professor, Department of Pediatric Dentistry, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Assistant Professor, Dental Sciences Research Center, Department of Pediatric Dentistry, School of Dentistry, Guilan University of Medical Sciences, Rasht, Iran.
3- Professor, Department of Pediatric Dentistry, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Dentist, Tehran, Iran.
2- Assistant Professor, Dental Sciences Research Center, Department of Pediatric Dentistry, School of Dentistry, Guilan University of Medical Sciences, Rasht, Iran.
3- Professor, Department of Pediatric Dentistry, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Dentist, Tehran, Iran.
Full-Text [PDF 669 kb]
(692 Downloads)
| Abstract (HTML) (2512 Views)
Full-Text: (914 Views)
1. Introduction
Dental caries are among the most frequent chronic childhood diseases [1]. The most cost-effective way to prevent tooth decay is brushing with fluoridated toothpastes [2, 3].
Fluoride helps with the remineralization and reduces the cariogenic effect of bacteria on teeth [4]. In lower concentrations, fluoride enables the constant reposition of mineral compounds which are lost during acids attack on the enamel and fluorapatites formation, which are less susceptible to acids. Higher concentration of fluoride leads to calcium fluoride formation; i.e. a reservoir of fluoride [5]. Fluoride effect can be measured by microhardness level. There are several methods to measure it, such as microhardness changes, spectrophotometer, X-Ray, dye penetration, and densitometry [6, 7, 8].
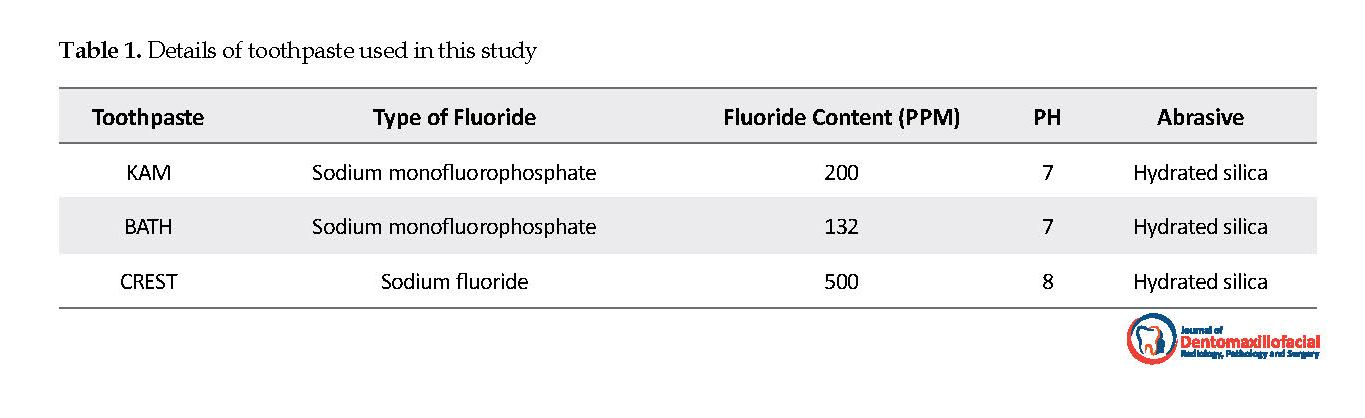
Different fluoride formulations, such as sodium fluoride, sodium monoflurophosphate, and amine fluoride are used as fluoride ions carriers in toothpastes. This in vitro study was designed to compare the surface microhardness changes of enamel, following the use of KAM (MFP, 200 ppm), BATH (MFP, 132 ppm), and CREST (NaF, 500 ppm) pediatric toothpaste.
2. Materials and Methods
This in vitro experimental study was performed on 45 sound primary molars extracted due to orthodontic purposes. The study samples were selected through convenience sampling method. The study samples were stored in tapped water at room temperature. They were polished with pumice and distilled water for 10 seconds; then, mounted in a particular transparent self-cured acrylic frame. To evaluate the transparent appearance of enamel surfaces, we examined surfaces under light microscope (Siemens, Germany) using X 100 objective lens. Buccal surfaces of the teeth were used, and other surfaces were covered with nail polish. Microhardness was measured by Vickers microhardness instrument (made in, Germany Gmbh), in three steps.
Dental caries are among the most frequent chronic childhood diseases [1]. The most cost-effective way to prevent tooth decay is brushing with fluoridated toothpastes [2, 3].
Fluoride helps with the remineralization and reduces the cariogenic effect of bacteria on teeth [4]. In lower concentrations, fluoride enables the constant reposition of mineral compounds which are lost during acids attack on the enamel and fluorapatites formation, which are less susceptible to acids. Higher concentration of fluoride leads to calcium fluoride formation; i.e. a reservoir of fluoride [5]. Fluoride effect can be measured by microhardness level. There are several methods to measure it, such as microhardness changes, spectrophotometer, X-Ray, dye penetration, and densitometry [6, 7, 8].
Different fluoride formulations, such as sodium fluoride, sodium monoflurophosphate, and amine fluoride are used as fluoride ions carriers in toothpastes. This in vitro study was designed to compare the surface microhardness changes of enamel, following the use of KAM (MFP, 200 ppm), BATH (MFP, 132 ppm), and CREST (NaF, 500 ppm) pediatric toothpaste.
2. Materials and Methods
This in vitro experimental study was performed on 45 sound primary molars extracted due to orthodontic purposes. The study samples were selected through convenience sampling method. The study samples were stored in tapped water at room temperature. They were polished with pumice and distilled water for 10 seconds; then, mounted in a particular transparent self-cured acrylic frame. To evaluate the transparent appearance of enamel surfaces, we examined surfaces under light microscope (Siemens, Germany) using X 100 objective lens. Buccal surfaces of the teeth were used, and other surfaces were covered with nail polish. Microhardness was measured by Vickers microhardness instrument (made in, Germany Gmbh), in three steps.
The study samples were randomly assigned into three groups, as follows: BATH, KAM, and CREST toothpaste groups. The detailed information of toothpaste types is presented in Table 1. The microhardness of samples was also measured. A force of 200 g/10 secs was applied in three-point distances of 500, 1000, 1500 micrometers on each sample. Furthermore, the mean value of the three points was obtained as the standard reading. Then, each sample was primarily immersed in to 5m of 1% stirred citric acid for 6 minutes. Next, they were immersed into 10 mL of 1% unstirred citric acid for 15 minutes. After that, microhardness was re-measured.
In the third step, after measuring the PH of toothpaste, the samples were inserted in the suspension of toothpaste (5 g toothpaste +10 mL artificial saliva) for 2 minutes. The daily cycling regimen comprised of 3x1 min acid challenges and 2x2 min treatment periods. After rinsing with distilled water and replacing into artificial saliva, microhardness was remeasured 10 days later by a person who was blind to teeth classification. Analysis of Variance (ANOVA) and Tukey’s test was used for data analysis.
3. Results
Demineralization decreased the surface microhardness of enamel (P=0.001). After exposure to the suspension of toothpaste, the highest and lowest microhardness levels belonged to KAM and CREST toothpaste, respectively (Table 2). We applied ANOVA to evaluate the changes in surface microhardness in three groups. The relevant data suggested a significant increase in microhardness 10 days after intervention (step 3), compared to the demineralization phase (step 2) in all groups (P=0.001) (Table 2). We used Tukey’s test for paired group comparison. The obtained data revealed no significant difference between surface microhardness produced by the three different toothpastes (Table 3).
4. Discussion
The study results indicated that the greatest and lowest recovery of microhardness belonged to KAM and CREST toothpastes, respectively; however, this difference was not significant.
In this study, intact primary molars were collected from Tehran residents in district 2. Because of the difference in dental composition and their effect on fluoride absorption rate, it was better to select the samples from a similar region. In other words, people’s residential location and drinking water fluoride content must have been controlled.
We only considered an aspect of the buccal surface of each tooth; because of the treatment limitations on a particular surface in each tooth might eliminate the disrupting effects [9, 10].
In the current study, the studied teeth were preserved in tap water, and no antiseptic solution was used; because chemical compounds affect the enamel microhardness, as confounding factors [11]. In the study by Saliva et al. , preserving samples in 0.01% thymol solution and 2% formaldehyde resulted in the microscopic changes of tooth structure, and the enamel became more susceptible to demineralization [12].
In the study by Lee et al. crowns were separated from root by diamond rotary disk [3]. In this method, the stress of cutting could be a confounding factor and might affect the mechanical properties of enamel. Thus, we did not separate the crown from root.
Haung et al. used enamel blocks without crown support. In the Vickers method, force is applied to samples; then, microhardness is measured [13]. It is expected that these forces be better-tolerated if samples are equipped with crown support.
Wefel et al. argued that acidic fluoride causes the further enhancement of enamel microhardness compared to non-acidic fluoride [14]. Jabbari, far et al. used non-acidic toothpaste with equal pH [15]. In the current study, similarly, non-acidic toothpaste was used. The pH of BATH and KAM was 7, and the pH of CREST was 8.
In the current study, comparing KAM (MFP,200 ppm) and BATH (MFP,132 ppm) toothpaste, KAM toothpaste, with higher fluoride concentration resulted in higher increase of microhardness. Additionally, KAM (MFP,200 ppm) and BATH (MFP,132 ppm) toothpaste increased microhardness more than CREST (NaF,500 ppm), toothpaste. This is probably due to the interaction of ingredients, inconsistent distribution, solubility of fluoride, and different pH values of studied toothpaste [15].
Chaudhary et al.suggested that fluoride-free toothpaste increased microhardness more than those with monofluorophosphate [10].
Jabbarifar et al. found no significant difference in microhardness changes after applying CREST (500 ppm), POUNE (500 ppm), and fluoride-free POUNE toothpastes [15]. CREST toothpaste (1100 ppm) increased microhardness more than CREST (500 ppm) and POUNE (500 ppm).
According to Casal et al. the effect of 1400 ppm toothpaste, compared to fluoride-free toothpaste was not statistically significant due to inappropriate fluoride release [16]. Doga et al. revealed that 226 ppm toothpastes increased enamel remineralization more than 450 ppm and 900 ppm toothpastes [17].
Craig et al. documented that a sodium fluoride toothpaste (1150 ppm) increased microhardness more than Crest toothpaste (sodium fluoride 1100 ppm) and placebo [18]. Sodium fluoride toothpastes (1450 ppm) increased microhardness more than Eelmex sensitive toothpaste (1450 ppm) and placebo.
A limitation to the present study was that other toothpaste ingredients might lead to variations in the surface microhardness of enamel. Inappropriate conditions, like components of natural saliva and oral cavity were other limitations of the study.
5. Conclusion
The study results suggested no significant difference in microhardness changes after applying KAM, BATH, and CREST toothpaste. Therefore, using Iranian-made pediatric kinds of toothpaste, which are inexpensive and have lower concentration of fluoride are recommended.
Ethical Considerations
Compliance with ethical guidelines
There is no ethical principle to be considered doing this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors.
Authors contribution's
The authors declared no conflict of interest.
Conflict of interest
There are no conflicts of interest to be declared.
Acknowledgements
The authors would like to thank the Department of Pediatric Dentistry of Shahid Beheshti University of Medical Sciences.
References
McDonald RE, Avery DR. Dentistry for the child and adolescent. Mosby: Elsevier; 2004.
Harris NO, Garcia-Godoy F. Primary preventive dentistry. Upper Saddle River, New Jersey: Pearson Education; 2004.
Nassar HM, Lippert F, Eckert GJ, Hara AT. Impact of toothbrushing frequency and toothpaste fluoride/abrasivity levels on incipient artificial caries lesion abrasion. Journal of Dental Research. 2018; 76:89-92. [DOI:10.1016/j.jdent.2018.06.018] [PMID]
Horst JA, Tanzer JM, Milgrom PM. Fluorides and other preventive strategies for tooth decay. Dental Clinics of North America. 2018; 62(2):207-34. [DOI:10.1016/j.cden.2017.11.003] [PMID] [PMCID]
Zębów ZF, Młodzieży UD, Polskiego PP. Fluoride compounds in dental caries prophylaxis in children and adolescents–review of polish literature. Przegląd Epidemiologiczny. 2017; 71(4):603-11.
White DJ. The application of in vitro models to research on demineralization and remineralization of the teeth. Advances in Dental Research. 1995; 9(3):175-93. [DOI:10.1177/08959374950090030101] [PMID]
Cate JT. In vitro studies on the effects of fluoride on de-and remineralization. Journal of Dental Research. 1990; 69(2):614-9. [DOI:10.1177/00220345900690S120] [PMID]
Rana R, Itthagarun A, King NM. Effects of dentifrices on artificial caries like lesions: An invitro PH cycling study. International Dental Journal. 2007; 57(4):243-8. [DOI:10.1111/j.1875-595X.2007.tb00127.x] [PMID]
Peres KG, Armenio MF, Peres MA, Traebert J, De Lacerda JT. Dental erosion in 12 year old school child: A cross-sectional study in southern brazil. International Journal of Paediatric Dentistry. 2005; 15(4):249-55. [DOI:10.1111/j.1365-263X.2005.00643.x] [PMID]
Chaudhary A, Ingle NA, Kaur N, Gupta R. Effect of fluoridated dentifrices on microhardness of enamel surface: In vitro study. Journal of Advanced Oral Research. 2013; 4(1):11-6. [DOI:10.1177/2229411220130103]
Amaechi BT, Higham SM, Edgar WM. Efficacy of sterilization methods and their effect on enamel demineralization. Caries Research. 1998; 32(6):441-46. [DOI:10.1159/000016485] [PMID]
Moura JS, Rodrigues LK, Del Bel Cury AA, Lima EM, Garcia RM. Influence of storage solution on enamel demineralization submitted to pH cycling. Journal of Applied Oral Science: Revista FOB . 2004; 12(3):205-8. [DOI:10.1590/S1678-77572004000300008] [PMID]
Huang SB, Gao SS, Yu HY. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed Mater. 2009; 4(3):1748-53. [DOI:10.1088/1748-6041/4/3/034104] [PMID]
Thaveesangpanich P, Itthagarun A, King NM, Wefel JS, Tay FR. In vitro model for evaluation the effect of child formula toothpaste on artificial caries in primary dentition enamel. The American Journal of Dental Science. 20051; 18(3):212-6.
Jabbarifar E, Salavati SH, Khosravi K, Tavakoli N. [Microhardness changes in primary tooth enamel following application of crest and pooneh pediatric fluoride toothpaste (In vitro survey) (Persian)]. Journal of Mashhad Dental School. 2010; 33(4):277-84.
Casals E, Boukpessi PT, Mc Queen CM, Eversole SL, Faller RV. Anti caries potential of commercial dentifrices as determind by fluoridation and remineralization efficiency. The Journal of Contemporary Dental Practice. 2007; 8(7):1-19. [DOI:10.5005/jcdp-8-7-1] [PMID]
Doga F, Civelek A, Oktay I. Effect of different fluoride concentration on remineralization of demineralization enamel: An in vitro PH cycling. Journal of Oral Health and Dental Management. 2004; 3(1):20-6.
Newby CS, Creeth JE, Rees GD, Schemehorn BR. Surface microhardness changes, enamel fluoride uptake, and fluoride availability from commercial toothpastes. The Journal of Clinical Dentistry. 2006; 17(4):94-9.
3. Results
Demineralization decreased the surface microhardness of enamel (P=0.001). After exposure to the suspension of toothpaste, the highest and lowest microhardness levels belonged to KAM and CREST toothpaste, respectively (Table 2). We applied ANOVA to evaluate the changes in surface microhardness in three groups. The relevant data suggested a significant increase in microhardness 10 days after intervention (step 3), compared to the demineralization phase (step 2) in all groups (P=0.001) (Table 2). We used Tukey’s test for paired group comparison. The obtained data revealed no significant difference between surface microhardness produced by the three different toothpastes (Table 3).
4. Discussion
The study results indicated that the greatest and lowest recovery of microhardness belonged to KAM and CREST toothpastes, respectively; however, this difference was not significant.
In this study, intact primary molars were collected from Tehran residents in district 2. Because of the difference in dental composition and their effect on fluoride absorption rate, it was better to select the samples from a similar region. In other words, people’s residential location and drinking water fluoride content must have been controlled.
We only considered an aspect of the buccal surface of each tooth; because of the treatment limitations on a particular surface in each tooth might eliminate the disrupting effects [9, 10].
In the current study, the studied teeth were preserved in tap water, and no antiseptic solution was used; because chemical compounds affect the enamel microhardness, as confounding factors [11]. In the study by Saliva et al. , preserving samples in 0.01% thymol solution and 2% formaldehyde resulted in the microscopic changes of tooth structure, and the enamel became more susceptible to demineralization [12].
In the study by Lee et al. crowns were separated from root by diamond rotary disk [3]. In this method, the stress of cutting could be a confounding factor and might affect the mechanical properties of enamel. Thus, we did not separate the crown from root.
Haung et al. used enamel blocks without crown support. In the Vickers method, force is applied to samples; then, microhardness is measured [13]. It is expected that these forces be better-tolerated if samples are equipped with crown support.
Wefel et al. argued that acidic fluoride causes the further enhancement of enamel microhardness compared to non-acidic fluoride [14]. Jabbari, far et al. used non-acidic toothpaste with equal pH [15]. In the current study, similarly, non-acidic toothpaste was used. The pH of BATH and KAM was 7, and the pH of CREST was 8.
In the current study, comparing KAM (MFP,200 ppm) and BATH (MFP,132 ppm) toothpaste, KAM toothpaste, with higher fluoride concentration resulted in higher increase of microhardness. Additionally, KAM (MFP,200 ppm) and BATH (MFP,132 ppm) toothpaste increased microhardness more than CREST (NaF,500 ppm), toothpaste. This is probably due to the interaction of ingredients, inconsistent distribution, solubility of fluoride, and different pH values of studied toothpaste [15].
Chaudhary et al.suggested that fluoride-free toothpaste increased microhardness more than those with monofluorophosphate [10].
Jabbarifar et al. found no significant difference in microhardness changes after applying CREST (500 ppm), POUNE (500 ppm), and fluoride-free POUNE toothpastes [15]. CREST toothpaste (1100 ppm) increased microhardness more than CREST (500 ppm) and POUNE (500 ppm).
According to Casal et al. the effect of 1400 ppm toothpaste, compared to fluoride-free toothpaste was not statistically significant due to inappropriate fluoride release [16]. Doga et al. revealed that 226 ppm toothpastes increased enamel remineralization more than 450 ppm and 900 ppm toothpastes [17].
Craig et al. documented that a sodium fluoride toothpaste (1150 ppm) increased microhardness more than Crest toothpaste (sodium fluoride 1100 ppm) and placebo [18]. Sodium fluoride toothpastes (1450 ppm) increased microhardness more than Eelmex sensitive toothpaste (1450 ppm) and placebo.
A limitation to the present study was that other toothpaste ingredients might lead to variations in the surface microhardness of enamel. Inappropriate conditions, like components of natural saliva and oral cavity were other limitations of the study.
5. Conclusion
The study results suggested no significant difference in microhardness changes after applying KAM, BATH, and CREST toothpaste. Therefore, using Iranian-made pediatric kinds of toothpaste, which are inexpensive and have lower concentration of fluoride are recommended.
Ethical Considerations
Compliance with ethical guidelines
There is no ethical principle to be considered doing this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors.
Authors contribution's
The authors declared no conflict of interest.
Conflict of interest
There are no conflicts of interest to be declared.
Acknowledgements
The authors would like to thank the Department of Pediatric Dentistry of Shahid Beheshti University of Medical Sciences.
References
McDonald RE, Avery DR. Dentistry for the child and adolescent. Mosby: Elsevier; 2004.
Harris NO, Garcia-Godoy F. Primary preventive dentistry. Upper Saddle River, New Jersey: Pearson Education; 2004.
Nassar HM, Lippert F, Eckert GJ, Hara AT. Impact of toothbrushing frequency and toothpaste fluoride/abrasivity levels on incipient artificial caries lesion abrasion. Journal of Dental Research. 2018; 76:89-92. [DOI:10.1016/j.jdent.2018.06.018] [PMID]
Horst JA, Tanzer JM, Milgrom PM. Fluorides and other preventive strategies for tooth decay. Dental Clinics of North America. 2018; 62(2):207-34. [DOI:10.1016/j.cden.2017.11.003] [PMID] [PMCID]
Zębów ZF, Młodzieży UD, Polskiego PP. Fluoride compounds in dental caries prophylaxis in children and adolescents–review of polish literature. Przegląd Epidemiologiczny. 2017; 71(4):603-11.
White DJ. The application of in vitro models to research on demineralization and remineralization of the teeth. Advances in Dental Research. 1995; 9(3):175-93. [DOI:10.1177/08959374950090030101] [PMID]
Cate JT. In vitro studies on the effects of fluoride on de-and remineralization. Journal of Dental Research. 1990; 69(2):614-9. [DOI:10.1177/00220345900690S120] [PMID]
Rana R, Itthagarun A, King NM. Effects of dentifrices on artificial caries like lesions: An invitro PH cycling study. International Dental Journal. 2007; 57(4):243-8. [DOI:10.1111/j.1875-595X.2007.tb00127.x] [PMID]
Peres KG, Armenio MF, Peres MA, Traebert J, De Lacerda JT. Dental erosion in 12 year old school child: A cross-sectional study in southern brazil. International Journal of Paediatric Dentistry. 2005; 15(4):249-55. [DOI:10.1111/j.1365-263X.2005.00643.x] [PMID]
Chaudhary A, Ingle NA, Kaur N, Gupta R. Effect of fluoridated dentifrices on microhardness of enamel surface: In vitro study. Journal of Advanced Oral Research. 2013; 4(1):11-6. [DOI:10.1177/2229411220130103]
Amaechi BT, Higham SM, Edgar WM. Efficacy of sterilization methods and their effect on enamel demineralization. Caries Research. 1998; 32(6):441-46. [DOI:10.1159/000016485] [PMID]
Moura JS, Rodrigues LK, Del Bel Cury AA, Lima EM, Garcia RM. Influence of storage solution on enamel demineralization submitted to pH cycling. Journal of Applied Oral Science: Revista FOB . 2004; 12(3):205-8. [DOI:10.1590/S1678-77572004000300008] [PMID]
Huang SB, Gao SS, Yu HY. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed Mater. 2009; 4(3):1748-53. [DOI:10.1088/1748-6041/4/3/034104] [PMID]
Thaveesangpanich P, Itthagarun A, King NM, Wefel JS, Tay FR. In vitro model for evaluation the effect of child formula toothpaste on artificial caries in primary dentition enamel. The American Journal of Dental Science. 20051; 18(3):212-6.
Jabbarifar E, Salavati SH, Khosravi K, Tavakoli N. [Microhardness changes in primary tooth enamel following application of crest and pooneh pediatric fluoride toothpaste (In vitro survey) (Persian)]. Journal of Mashhad Dental School. 2010; 33(4):277-84.
Casals E, Boukpessi PT, Mc Queen CM, Eversole SL, Faller RV. Anti caries potential of commercial dentifrices as determind by fluoridation and remineralization efficiency. The Journal of Contemporary Dental Practice. 2007; 8(7):1-19. [DOI:10.5005/jcdp-8-7-1] [PMID]
Doga F, Civelek A, Oktay I. Effect of different fluoride concentration on remineralization of demineralization enamel: An in vitro PH cycling. Journal of Oral Health and Dental Management. 2004; 3(1):20-6.
Newby CS, Creeth JE, Rees GD, Schemehorn BR. Surface microhardness changes, enamel fluoride uptake, and fluoride availability from commercial toothpastes. The Journal of Clinical Dentistry. 2006; 17(4):94-9.
Type of Study: Original article |
Received: 2018/07/23 | Accepted: 2018/11/11 | Published: 2018/12/1
Received: 2018/07/23 | Accepted: 2018/11/11 | Published: 2018/12/1
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |