Thu, Apr 25, 2024
Volume 6, Issue 4 (12-2017)
2017, 6(4): 103-114 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hashemipour M A, Abdi F, Teymori M, Zeraat Pisheh M, Samie Rad S. Effects of Cigarette Smoke, Alcohol, Vitamin E and Vitamin C on Oral Mucosa and Salivary Peroxides Enzyme in Rats. Journal title 2017; 6 (4) :103-114
URL: http://3dj.gums.ac.ir/article-1-292-en.html
URL: http://3dj.gums.ac.ir/article-1-292-en.html
Maryam Alsadat Hashemipour * 

 1, Fatemeh Abdi2
1, Fatemeh Abdi2 

 , Maryam Teymori3
, Maryam Teymori3 

 , Mohsen Zeraat Pisheh4
, Mohsen Zeraat Pisheh4 

 , Sahand Samie Rad5
, Sahand Samie Rad5 



 1, Fatemeh Abdi2
1, Fatemeh Abdi2 

 , Maryam Teymori3
, Maryam Teymori3 

 , Mohsen Zeraat Pisheh4
, Mohsen Zeraat Pisheh4 

 , Sahand Samie Rad5
, Sahand Samie Rad5 

1- Associate Professor, Department of Oral & Maxillofacial Medicine, Dental & Oral Disease Research Center, Kerman University of Medical Sciences, Kerman, Iran. , m_s_hashemipour@yahoo.com
2- DDS. Dentist.
3- Dental Student. School of Dentistry, Kerman University of Medical Sciences, Kerman, Iran.
4- Dentist, Department of Oral Medicine, Faculty of Dentistry, Kerman University of Medical Sciences, Kerman, Iran.
5- Dental Student, Department of Oral Medicine, Faculty of Dentistry, Kerman University of Medical Sciences, Kerman, Iran.
2- DDS. Dentist.
3- Dental Student. School of Dentistry, Kerman University of Medical Sciences, Kerman, Iran.
4- Dentist, Department of Oral Medicine, Faculty of Dentistry, Kerman University of Medical Sciences, Kerman, Iran.
5- Dental Student, Department of Oral Medicine, Faculty of Dentistry, Kerman University of Medical Sciences, Kerman, Iran.
Full-Text [PDF 895 kb]
(1003 Downloads)
| Abstract (HTML) (2993 Views)
Full-Text: (1081 Views)
1. Introduction
Oral cancer is the fourth and sixth common cancer in men and women, respectively. It is one of the most malignancy in developing countries. This cancer accounts for 50% of deadly cancers. Men’s involvement is three times more than women and usually occurs after 40 years old. Mortality rate of this cancer has increased in almost all of the European countries over the past decades.
Although diagnostic and therapeutic equipment have advanced significantly, the survival rate is not satisfying yet. Because most oral cancers are diagnosed in their advanced stage, the prognosis are usually poor [1-5]. The most important risk factors for this cancer are smoking (tobacco, cigarette, pipe, snuff) and alcoholic drinks. The main cause of oral cancer is tobacco use which is responsible for 50% to 90% of new cases in the world. The prevalence of oral cancer in smokers is 4-7 times more than nonsmokers and the average age of disease onset in smokers is 15 years earlier than nonsmokers [1, 6].
According to the most recent studies, cigarette smoking increases the risk of oral cancer because of destroying protective molecules in oral saliva and transform it to a dangerous environment. Cigarette smoke compounds are not only harmful per se but also make body to act against itself [1, 6, 7]. Moreover, free oxygen radicals and nitrogen in inhaled smoke can induce cancer process [3]. Oral saliva contains antioxidants; molecules which naturally protect the body against cancer, but the research has shown that cigarette smoke destroys these molecules and transforms saliva to dangerous compounds. Research studies show that healthy saliva (under the effect of smoking) not only loses its useful properties but destroys oral cells too [1, 6, 7].
Field cancerization theory is recently accepted regarding the carcinogenic effect of cigarette in oral mucosa. According to this theory, substances from cigarette smoke directly and constantly assault epithelial cells and gradually transform them to malignant cells. These transformations are due to various reactive oxygen species and active nitrogen species in cigarette smoke. Initially, they cause dysplastic changes in mucosa and then turn into carcinoma in situ. Finally, there will be infiltration and oral cancer metastasis [8]. Also alcohol is considered as a possible risk factor for oral cancer. After using alcohol, staldehyde is produced locally by oral microflora and ethanol is transformed to staldehyde oxides [9].Saliva is the first biological fluid that contacts the cigarette smoke. Saliva protects against harmful agents like microorganisms, toxins, and various oxidants. It is postulated that cigarette smoke can reduce peroxides enzyme activity in plasma and saliva. Peroxides system of the oral cavity mainly consists of peroxides and myeloperoxides enzymes secreted from major salivary glands mainly parotid. On the whole, peroxides enzyme participates in 80% of oral peroxides activity. However, myeloperoxides produced by leucocytes in inflammatory regions are responsible for 20% of oral oxides activity [6]. Free oxygen radicals play an important role in pathogeneses of different diseases like cancer.
Antioxidant enzymes, synergistic with each other detoxify lipids peroxidation effect. Increase in lipid peroxidation together with venous blood antioxidant decrease have been reported in patients with oral Squamous Cell Carcinoma (SCC) [9]. Although antibacterial effects of peroxides is under research, its possible anticancer role against the most common and deadly SCC has rarely been reported in scientific articles and case reports [6].
Also studies have shown that eating lots of fruits, decreases the risk of cancers like digestive system cancers. Based on epidemiological research, consuming lots of fruits especially sour fruits and juice decreases the risk of precancerous lesions in male digestive system. Many years of research on more than 42000 men shows that using enough fruit and vegetable in a healthy diet decreases the risk of men’s precancerous lesions in digestive system around 30% to 40% [8, 10-14]. As there is no research on antioxidants effects (which their main ones are vitamin C and E) on oral mucosa changes in smokers and alcohol users, we aimed to examine the effects of cigarette smoke, alcohol, vitamin E and vitamin C use on oral mucosa and salivary peroxides enzyme in rats.
2. Materials and Methods
Study animals
This study is a laboratory experiment. In this study, 128 seven-week-old male Wister rats weighing 200 to 250 g were used. They were kept in plastic cages (with ad libitum access to food and water). All of the animals spent one week in the laboratory before the exposure to get accustomed to the laboratory environment [15]. Then, they were divided into 16 groups (4 rats died during the study which were replaced).
The first group were exposed to cigarette, the second group to alcohol, the third group to vitamin C, the fourth group to vitamin E, the fifth group to alcohol and cigarette, the sixth group to cigarette and vitamin E, the seventh group to cigarette and vitamin C, the eighth group to alcohol and vitamin C, the ninth group to alcohol and vitamin E, the 10th group to vitamin E and C, the 11th group to cigarette, alcohol, and vitamin E, the 12th group to cigarette, alcohol, and vitamin C, the 13th group to cigarette, vitamin E, and C, the 14th group to alcohol, vitamin E, and C, the 15th group to cigarette, alcohol, vitamin E, and C, and the 16th group were control. All animals were weighted (scaled) in days 1, 15, 30, 45, 60 before collecting saliva and after anesthesia.
Exposure to cigarette protocol
Exposure to cigarette was done based on Nogueira-Filho et al. protocol [16]. To this end, a cigarette smoke making machine was designed and registered at intellectual property center. The rats belonged to groups of alcohol alone; cigarette alone; cigarette and vitamin E; cigarette and vitamin C; cigarette, alcohol and vitamin E; cigarette, alcohol, and vitamin C; cigarette, vitamin E, and vitamin C; cigarette, alcohol, vitamin E, and C; were placed in the exposure box for 20 minutes per day and at the end of 60th days, they were killed.
This machine consists of a glass box with 0.6×1.5×1 m³ in dimensions, 2 air pumps and 4 inflow tubes. At the same time, the animals were put into the box and the smoke of 12 (Bahman brand) cigarettes containing 104 g nicotine and 17 mg fibers was puffed into the box. The animals were forced to breathe tobacco containing air for 20 minutes each day during 60 days. The roof was completely sealed and there was no outgoing way for smoke [16].
Alcohol exposure protocol
We used 36% ethanol with 0.08 concentration in this research. This amount was added to consumed water of the rats belonged to alcohol, alcohol and cigarette, and alcohol and antioxidant substances (Vitamin E and vitamin C) each day. At the end of 60 days, the rats’ salivary glands were studied for alcohol effects [17,18]. Also staldehyde was studied in days 1, 15, 30, 45, and 60.
Antioxidant substances exposure protocol (vitamins E and C)
Vitamin E was prepared in dl-alpha-tocopheryl acetate (Sigma Company) form , 250 mg/kg, and was added to the water of rats belonged to alcohol and antioxidants, cigarette and antioxidants, and antioxidants group each day [19]. Vitamin C, 25 mg/kg, was also added to animals’ water [20].
Saliva collection
All the animals’ saliva was collected and measured on the day 0, 15, 30, 45, and 60. The rats were anesthetized by ketamine, 60 mg/kg and rampond (muscle relaxant), 7.5 mg/kg. Then rats’ saliva was measured with placing cotton ball (that was weighted before) for 15 minutes. After 15 minutes, the cotton ball was measured again and the difference was reported as saliva amount in 15 minutes [21].
Measurement of salivary proteins and peroxides enzyme
The collected saliva from the animals in cotton ball was discharged into sterile urine tubes. Then the samples were transported to pathology laboratory and were kept under 18˚C in a freezing condition. To measure salivary peroxides enzyme activity rate and total protein concentration, at first the frozen samples were placed at the laboratory temperature for half an hour, then they were centrifuged at 3500 rpm for 20 minutes and the obtained surface liquid was transported to Ependr microsampler with suitable volume samplers. Every sample’s feature was written on the microtube. Afterwards, the total protein concentration was measured with Lowry method in comparison with bovine serum albumin as standard.
Salivary peroxides enzyme was measured by using Tetramethylbenzedine (TMB). In this method, saliva was diluted 1:10 by physiologic serum and 20 mL was placed in a 20 squares microplate. About 89 µL of solution that consists of TMB was added to each square and the solution was put in darkness, at room temperature for 30 minutes. Each microplate’s color activity was evaluated by 100 µL of 0.6% sulfuric acid in 450 nm photo absorption by ELIZA method [9].
Histological study of salivary glands and oral mucosa
Animals’ parotid glands were excised at the end of 60 days after killing with deep anesthesia. Then every sample was placed in formaldehyde (diluted with 1 to 10 ratio) and sent to the pathology laboratory. In the laboratory, the samples were stained with Hematoxylin-Eosin (H&E) after fixation and were studied for vessel dilation, hyperemia, inflammatory cells, intercalated duct portion, striated duct portion, and vacuolar degeneration [9]. Also a sample of tongue’s lateral borders was biopsied (because of being high risk and precancerous changes) in day 60, fixed in 10% formalin, sent to a pathology laboratory and after diving in paraffin and H&E staining, they were studied by a pathologist who was blinded to the experiment (Table 1) [22].
The obtained data were analyzed by Student t test, ANOVA, and Welch test in SPSS v.17. P value equal or less than 0.05 was considered significant. All study procedure was performed in compliance with international guidelines on using animals and under control of Medical University Ethics Committee (KA=124).
3. Results
The animals were studied with regard to their weight, salivary glands pathologic changes, tongue lateral border malignant changes, saliva secretion rate, salivary peroxides enzyme rate and saliva’s staldehyde. This study showed that body weight of groups had a significant rise compared to the control group. However, there was a significant weight decrease in other groups especially in groups 5 and 12 in comparison with the control group (Table 1).
Tongue lateral border pathologic changes
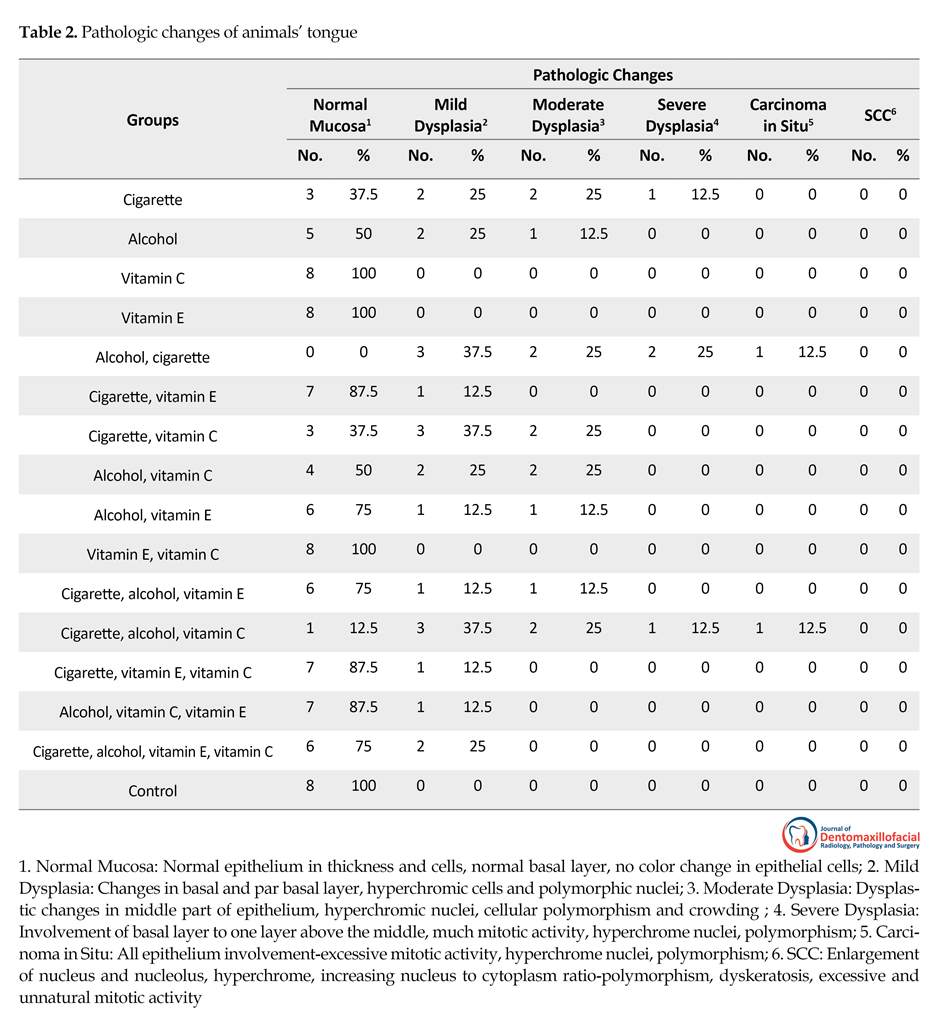
This study showed that cigarette consumption had changed rats’ mucosa from mild to severe dysplasia. So that only 3 rats (37.5%) in the first group had a normal mucosa. Also alcohol consumption in the second group had caused mild to moderate dysplastic changes. In groups number 5 and 12 that had simultaneous consumption of alcohol and cigarette, severe pathologic changes were detected. So that one carcinoma in situ was reported in each group (12.5% and none of the animals had a normal mucosa in group 5).
In groups consuming vitamin E and vitamin C (groups 3, 4, and 10), no pathologic changes were observed. Based on the study results, vitamin E inhibits pathologic changes due to cigarette and alcohol use in oral mucosa, so that vitamin E consumption in group number 6 (cigarettes plus Vitamin E) had caused significant pathologic changes compared to the cigarette only group. This vitamin had the same inhibiting effect on groups using alcohol and cigarette and alcohol alone. Although vitamin C decreased malignant changes, its effect was not as strong as vitamin E and the difference was significant (P=0.03) (Table 2).
As seen in Table 3, cigarette and alcohol use cause changes like vessels dilation, hyperemia, inflammatory cells increase, and intercalated duct portion in parotid gland. No histological changes were seen in salivary glands of groups using vitamin C or vitamin E as well as the control group. This study showed that using vitamin
Oral cancer is the fourth and sixth common cancer in men and women, respectively. It is one of the most malignancy in developing countries. This cancer accounts for 50% of deadly cancers. Men’s involvement is three times more than women and usually occurs after 40 years old. Mortality rate of this cancer has increased in almost all of the European countries over the past decades.
Although diagnostic and therapeutic equipment have advanced significantly, the survival rate is not satisfying yet. Because most oral cancers are diagnosed in their advanced stage, the prognosis are usually poor [1-5]. The most important risk factors for this cancer are smoking (tobacco, cigarette, pipe, snuff) and alcoholic drinks. The main cause of oral cancer is tobacco use which is responsible for 50% to 90% of new cases in the world. The prevalence of oral cancer in smokers is 4-7 times more than nonsmokers and the average age of disease onset in smokers is 15 years earlier than nonsmokers [1, 6].
According to the most recent studies, cigarette smoking increases the risk of oral cancer because of destroying protective molecules in oral saliva and transform it to a dangerous environment. Cigarette smoke compounds are not only harmful per se but also make body to act against itself [1, 6, 7]. Moreover, free oxygen radicals and nitrogen in inhaled smoke can induce cancer process [3]. Oral saliva contains antioxidants; molecules which naturally protect the body against cancer, but the research has shown that cigarette smoke destroys these molecules and transforms saliva to dangerous compounds. Research studies show that healthy saliva (under the effect of smoking) not only loses its useful properties but destroys oral cells too [1, 6, 7].
Field cancerization theory is recently accepted regarding the carcinogenic effect of cigarette in oral mucosa. According to this theory, substances from cigarette smoke directly and constantly assault epithelial cells and gradually transform them to malignant cells. These transformations are due to various reactive oxygen species and active nitrogen species in cigarette smoke. Initially, they cause dysplastic changes in mucosa and then turn into carcinoma in situ. Finally, there will be infiltration and oral cancer metastasis [8]. Also alcohol is considered as a possible risk factor for oral cancer. After using alcohol, staldehyde is produced locally by oral microflora and ethanol is transformed to staldehyde oxides [9].Saliva is the first biological fluid that contacts the cigarette smoke. Saliva protects against harmful agents like microorganisms, toxins, and various oxidants. It is postulated that cigarette smoke can reduce peroxides enzyme activity in plasma and saliva. Peroxides system of the oral cavity mainly consists of peroxides and myeloperoxides enzymes secreted from major salivary glands mainly parotid. On the whole, peroxides enzyme participates in 80% of oral peroxides activity. However, myeloperoxides produced by leucocytes in inflammatory regions are responsible for 20% of oral oxides activity [6]. Free oxygen radicals play an important role in pathogeneses of different diseases like cancer.
Antioxidant enzymes, synergistic with each other detoxify lipids peroxidation effect. Increase in lipid peroxidation together with venous blood antioxidant decrease have been reported in patients with oral Squamous Cell Carcinoma (SCC) [9]. Although antibacterial effects of peroxides is under research, its possible anticancer role against the most common and deadly SCC has rarely been reported in scientific articles and case reports [6].
Also studies have shown that eating lots of fruits, decreases the risk of cancers like digestive system cancers. Based on epidemiological research, consuming lots of fruits especially sour fruits and juice decreases the risk of precancerous lesions in male digestive system. Many years of research on more than 42000 men shows that using enough fruit and vegetable in a healthy diet decreases the risk of men’s precancerous lesions in digestive system around 30% to 40% [8, 10-14]. As there is no research on antioxidants effects (which their main ones are vitamin C and E) on oral mucosa changes in smokers and alcohol users, we aimed to examine the effects of cigarette smoke, alcohol, vitamin E and vitamin C use on oral mucosa and salivary peroxides enzyme in rats.
2. Materials and Methods
Study animals
This study is a laboratory experiment. In this study, 128 seven-week-old male Wister rats weighing 200 to 250 g were used. They were kept in plastic cages (with ad libitum access to food and water). All of the animals spent one week in the laboratory before the exposure to get accustomed to the laboratory environment [15]. Then, they were divided into 16 groups (4 rats died during the study which were replaced).
The first group were exposed to cigarette, the second group to alcohol, the third group to vitamin C, the fourth group to vitamin E, the fifth group to alcohol and cigarette, the sixth group to cigarette and vitamin E, the seventh group to cigarette and vitamin C, the eighth group to alcohol and vitamin C, the ninth group to alcohol and vitamin E, the 10th group to vitamin E and C, the 11th group to cigarette, alcohol, and vitamin E, the 12th group to cigarette, alcohol, and vitamin C, the 13th group to cigarette, vitamin E, and C, the 14th group to alcohol, vitamin E, and C, the 15th group to cigarette, alcohol, vitamin E, and C, and the 16th group were control. All animals were weighted (scaled) in days 1, 15, 30, 45, 60 before collecting saliva and after anesthesia.
Exposure to cigarette protocol
Exposure to cigarette was done based on Nogueira-Filho et al. protocol [16]. To this end, a cigarette smoke making machine was designed and registered at intellectual property center. The rats belonged to groups of alcohol alone; cigarette alone; cigarette and vitamin E; cigarette and vitamin C; cigarette, alcohol and vitamin E; cigarette, alcohol, and vitamin C; cigarette, vitamin E, and vitamin C; cigarette, alcohol, vitamin E, and C; were placed in the exposure box for 20 minutes per day and at the end of 60th days, they were killed.
This machine consists of a glass box with 0.6×1.5×1 m³ in dimensions, 2 air pumps and 4 inflow tubes. At the same time, the animals were put into the box and the smoke of 12 (Bahman brand) cigarettes containing 104 g nicotine and 17 mg fibers was puffed into the box. The animals were forced to breathe tobacco containing air for 20 minutes each day during 60 days. The roof was completely sealed and there was no outgoing way for smoke [16].
Alcohol exposure protocol
We used 36% ethanol with 0.08 concentration in this research. This amount was added to consumed water of the rats belonged to alcohol, alcohol and cigarette, and alcohol and antioxidant substances (Vitamin E and vitamin C) each day. At the end of 60 days, the rats’ salivary glands were studied for alcohol effects [17,18]. Also staldehyde was studied in days 1, 15, 30, 45, and 60.
Antioxidant substances exposure protocol (vitamins E and C)
Vitamin E was prepared in dl-alpha-tocopheryl acetate (Sigma Company) form , 250 mg/kg, and was added to the water of rats belonged to alcohol and antioxidants, cigarette and antioxidants, and antioxidants group each day [19]. Vitamin C, 25 mg/kg, was also added to animals’ water [20].
Saliva collection
All the animals’ saliva was collected and measured on the day 0, 15, 30, 45, and 60. The rats were anesthetized by ketamine, 60 mg/kg and rampond (muscle relaxant), 7.5 mg/kg. Then rats’ saliva was measured with placing cotton ball (that was weighted before) for 15 minutes. After 15 minutes, the cotton ball was measured again and the difference was reported as saliva amount in 15 minutes [21].
Measurement of salivary proteins and peroxides enzyme
The collected saliva from the animals in cotton ball was discharged into sterile urine tubes. Then the samples were transported to pathology laboratory and were kept under 18˚C in a freezing condition. To measure salivary peroxides enzyme activity rate and total protein concentration, at first the frozen samples were placed at the laboratory temperature for half an hour, then they were centrifuged at 3500 rpm for 20 minutes and the obtained surface liquid was transported to Ependr microsampler with suitable volume samplers. Every sample’s feature was written on the microtube. Afterwards, the total protein concentration was measured with Lowry method in comparison with bovine serum albumin as standard.
Salivary peroxides enzyme was measured by using Tetramethylbenzedine (TMB). In this method, saliva was diluted 1:10 by physiologic serum and 20 mL was placed in a 20 squares microplate. About 89 µL of solution that consists of TMB was added to each square and the solution was put in darkness, at room temperature for 30 minutes. Each microplate’s color activity was evaluated by 100 µL of 0.6% sulfuric acid in 450 nm photo absorption by ELIZA method [9].
Histological study of salivary glands and oral mucosa
Animals’ parotid glands were excised at the end of 60 days after killing with deep anesthesia. Then every sample was placed in formaldehyde (diluted with 1 to 10 ratio) and sent to the pathology laboratory. In the laboratory, the samples were stained with Hematoxylin-Eosin (H&E) after fixation and were studied for vessel dilation, hyperemia, inflammatory cells, intercalated duct portion, striated duct portion, and vacuolar degeneration [9]. Also a sample of tongue’s lateral borders was biopsied (because of being high risk and precancerous changes) in day 60, fixed in 10% formalin, sent to a pathology laboratory and after diving in paraffin and H&E staining, they were studied by a pathologist who was blinded to the experiment (Table 1) [22].
The obtained data were analyzed by Student t test, ANOVA, and Welch test in SPSS v.17. P value equal or less than 0.05 was considered significant. All study procedure was performed in compliance with international guidelines on using animals and under control of Medical University Ethics Committee (KA=124).
3. Results
The animals were studied with regard to their weight, salivary glands pathologic changes, tongue lateral border malignant changes, saliva secretion rate, salivary peroxides enzyme rate and saliva’s staldehyde. This study showed that body weight of groups had a significant rise compared to the control group. However, there was a significant weight decrease in other groups especially in groups 5 and 12 in comparison with the control group (Table 1).
Tongue lateral border pathologic changes
This study showed that cigarette consumption had changed rats’ mucosa from mild to severe dysplasia. So that only 3 rats (37.5%) in the first group had a normal mucosa. Also alcohol consumption in the second group had caused mild to moderate dysplastic changes. In groups number 5 and 12 that had simultaneous consumption of alcohol and cigarette, severe pathologic changes were detected. So that one carcinoma in situ was reported in each group (12.5% and none of the animals had a normal mucosa in group 5).
In groups consuming vitamin E and vitamin C (groups 3, 4, and 10), no pathologic changes were observed. Based on the study results, vitamin E inhibits pathologic changes due to cigarette and alcohol use in oral mucosa, so that vitamin E consumption in group number 6 (cigarettes plus Vitamin E) had caused significant pathologic changes compared to the cigarette only group. This vitamin had the same inhibiting effect on groups using alcohol and cigarette and alcohol alone. Although vitamin C decreased malignant changes, its effect was not as strong as vitamin E and the difference was significant (P=0.03) (Table 2).
As seen in Table 3, cigarette and alcohol use cause changes like vessels dilation, hyperemia, inflammatory cells increase, and intercalated duct portion in parotid gland. No histological changes were seen in salivary glands of groups using vitamin C or vitamin E as well as the control group. This study showed that using vitamin
E and C has inhibiting effects and decreases histological changes seen in alcohol and cigarette using groups. Although Vitamin C decreased these changes, its effects was not like vitamin E, however, the difference was insignificant (P=0.12)
Saliva staldehyde changes in different groups
Figure 1 exhibits staldehyde amount in saliva in groups using alcohol alone or with cigarette, as well as vitamin E and vitamin C groups. As it is seen, this substance has increased in alcohol group gradually. Simultaneous use of alcohol and cigarette in group 5 has significantly increased this substance compared to alcohol alone group (P=0.02) and this could somehow indicates synergistic effects of cigarette and alcohol on this substance.
Vitamin E and Vitamin C caused gradual decrease of this substance in days 1 to 60 in groups under study and compared to alcohol group, this decrease was significant. The vitamin E role in decreasing this substance compared to vitamin C role in groups 8 and 9 (that only have used alcohol with these vitamins) was more significant and have caused marked staldehyde decrease.
Total saliva protein
Total saliva protein amount in cigarette and alcohol user groups (groups 1 and 2) decreased gradually within 60 days of study; however, this amount had not changed in groups 3, 4, 10 and the control group. The difference between groups 1 and 2 with control group with regard to decreasing total protein was significant (P=0.00) (Table 4).
Total saliva peroxides enzyme
There was a significant decrease in peroxides enzyme amount in groups 5 and 12 that had simultaneous use of cigarette and alcohol compared to the control group (P=0.00). There were not significant differences (P=0.14) between groups 5 and 12 and groups 1 and 2. This mean that simultaneous use of alcohol and cigarette lacked any significant effect on peroxides enzyme decrease. Table 5
Saliva staldehyde changes in different groups
Figure 1 exhibits staldehyde amount in saliva in groups using alcohol alone or with cigarette, as well as vitamin E and vitamin C groups. As it is seen, this substance has increased in alcohol group gradually. Simultaneous use of alcohol and cigarette in group 5 has significantly increased this substance compared to alcohol alone group (P=0.02) and this could somehow indicates synergistic effects of cigarette and alcohol on this substance.
Vitamin E and Vitamin C caused gradual decrease of this substance in days 1 to 60 in groups under study and compared to alcohol group, this decrease was significant. The vitamin E role in decreasing this substance compared to vitamin C role in groups 8 and 9 (that only have used alcohol with these vitamins) was more significant and have caused marked staldehyde decrease.
Total saliva protein
Total saliva protein amount in cigarette and alcohol user groups (groups 1 and 2) decreased gradually within 60 days of study; however, this amount had not changed in groups 3, 4, 10 and the control group. The difference between groups 1 and 2 with control group with regard to decreasing total protein was significant (P=0.00) (Table 4).
Total saliva peroxides enzyme
There was a significant decrease in peroxides enzyme amount in groups 5 and 12 that had simultaneous use of cigarette and alcohol compared to the control group (P=0.00). There were not significant differences (P=0.14) between groups 5 and 12 and groups 1 and 2. This mean that simultaneous use of alcohol and cigarette lacked any significant effect on peroxides enzyme decrease. Table 5
presents that vitamin C and E had protective effect and increased peroxides enzyme in groups 3, 4, and 10. Also these vitamins have relatively fixed peroxides enzyme amount in other groups under study.
4. Discussion
Oral cancer is the fourth and sixth common cancer in men and women, respectively. This cancer accounts for 50% of fatal cancers in India but only 4% in the Western countries. This cancer is more common in adults over 40 but its prevalence between teens who use chewing tobacco is rising. Men are affected to oral cancer four times more than women. Almost 400000 new cases of this disease are reported each year and most of them are from developing countries. Less than 50% of these patients survive 5 years after diagnosis.
Smoking tobacco products (nicotine, cigarette, pipe, snuff, tobacco), using alcohol drinks, iron and vitamin deficiency, syphilis, viral and fungal infections are some of the main causes of oral cancer. The most common sites of this cancer are lips and then tongue and floor of the mouth. Although oral cancer is very rare but it can
4. Discussion
Oral cancer is the fourth and sixth common cancer in men and women, respectively. This cancer accounts for 50% of fatal cancers in India but only 4% in the Western countries. This cancer is more common in adults over 40 but its prevalence between teens who use chewing tobacco is rising. Men are affected to oral cancer four times more than women. Almost 400000 new cases of this disease are reported each year and most of them are from developing countries. Less than 50% of these patients survive 5 years after diagnosis.
Smoking tobacco products (nicotine, cigarette, pipe, snuff, tobacco), using alcohol drinks, iron and vitamin deficiency, syphilis, viral and fungal infections are some of the main causes of oral cancer. The most common sites of this cancer are lips and then tongue and floor of the mouth. Although oral cancer is very rare but it can
destroy half of the face and sometimes it is necessary to remove mandible to save patient’s life [23].
This study was designed to determine the effects of alcohol and cigarette use on oral mucosa and saliva composition, also the inhibiting effects of vitamin E and C as two antioxidant substances. This research showed that using cigarette and alcohol separately and simultaneously causes pathologic changes in rat tongue from mild dysplasia to carcinoma in situ. This effect was more in simultaneous use of these two substances, in other words these substances have synergistic effects. Vitamin E and C decrease the pathologic effects of these two substances and a pretty more normal epithelium was reported in the rats using these two vitamins.
Rothman and Keller suggested a synergistic effect at simultaneous exposure of alcohol and cigarette. They also emphasized that every one of them alone would have certain effects on oral mucosa [24]. Other studies demonstrated the synergistic effect of cigarette and alcohol [25, 26]. Bross and Coombs showed that oral cancer appears 15 years earlier in women who were exposed to both alcohol and tobacco compared to women who were not using cigarette or alcohol. They noticed that cigarette alone has little effect in decreasing the age of oral cancer onset and alcohol exposure alone has no clear effect in decreasing the age of oral cancer onset [27]. Bandgarad et al. studied the alcohol and tobacco prognosis in oral cancer. They found that simultaneous use of alcohol and tobacco together or using tobacco alone affects the disease prognosis but using alcohol alone has no effect on prognosis [28].
This study was designed to determine the effects of alcohol and cigarette use on oral mucosa and saliva composition, also the inhibiting effects of vitamin E and C as two antioxidant substances. This research showed that using cigarette and alcohol separately and simultaneously causes pathologic changes in rat tongue from mild dysplasia to carcinoma in situ. This effect was more in simultaneous use of these two substances, in other words these substances have synergistic effects. Vitamin E and C decrease the pathologic effects of these two substances and a pretty more normal epithelium was reported in the rats using these two vitamins.
Rothman and Keller suggested a synergistic effect at simultaneous exposure of alcohol and cigarette. They also emphasized that every one of them alone would have certain effects on oral mucosa [24]. Other studies demonstrated the synergistic effect of cigarette and alcohol [25, 26]. Bross and Coombs showed that oral cancer appears 15 years earlier in women who were exposed to both alcohol and tobacco compared to women who were not using cigarette or alcohol. They noticed that cigarette alone has little effect in decreasing the age of oral cancer onset and alcohol exposure alone has no clear effect in decreasing the age of oral cancer onset [27]. Bandgarad et al. studied the alcohol and tobacco prognosis in oral cancer. They found that simultaneous use of alcohol and tobacco together or using tobacco alone affects the disease prognosis but using alcohol alone has no effect on prognosis [28].
According to the most recent studies, cigarette smoke increases the risk of oral cancer by destroying protective molecules in oral saliva and transforming it to a dangerous chemical compound. Also cigarette smoke is not only harmful, but it also causes the body to act against itself. Researchers have shown that saliva contains antioxidants, molecules that naturally protect the body against cancer. However, cigarette smoke destroys these molecules and transforms saliva to a dangerous compound. Also normal saliva not only loses its useful properties after exposure to cigarette but also destroys oral cells [29].
Studies have demonstrated that using lots of fruits decreases the risk of oral cancer. Using a lot of fruits especially sour fruits and citrus juice, decreases the risk of precancerous lesions formation and oral cancer in men. A Harvard University research on more than 42000 men shows that eating enough fruits and vegetables full of vitamin C reduces the risk of cancer up to 30% to 40% [29]. So far preventive effect of regular fruit and vegetable use has been demonstrated [30].
Vitamin E is a strong antioxidant. This vitamin could strengthen the immune system, improve cancer chemotherapy recovery, and reduce the side effects of radiotherapy and chemotherapy in patients with cancer. Investigations show that this vitamin has protective role with its preventive effect in breast, prostate, and urinary bladder cancers [30].
So far the effects of using vitamin E with alcohol and cigarette on malignant changes of oral mucosa have not been reported. In 1993, Garewal studied the published articles about beta carotene and vitamin E effects and their preventive role in developing oral cancer. The results showed that low consumption of beta carotene is associated with high risk of developing cancer. Also smokers have low beta carotene levels in oral mucosa cells compared to nonsmokers. In several laboratory
Studies have demonstrated that using lots of fruits decreases the risk of oral cancer. Using a lot of fruits especially sour fruits and citrus juice, decreases the risk of precancerous lesions formation and oral cancer in men. A Harvard University research on more than 42000 men shows that eating enough fruits and vegetables full of vitamin C reduces the risk of cancer up to 30% to 40% [29]. So far preventive effect of regular fruit and vegetable use has been demonstrated [30].
Vitamin E is a strong antioxidant. This vitamin could strengthen the immune system, improve cancer chemotherapy recovery, and reduce the side effects of radiotherapy and chemotherapy in patients with cancer. Investigations show that this vitamin has protective role with its preventive effect in breast, prostate, and urinary bladder cancers [30].
So far the effects of using vitamin E with alcohol and cigarette on malignant changes of oral mucosa have not been reported. In 1993, Garewal studied the published articles about beta carotene and vitamin E effects and their preventive role in developing oral cancer. The results showed that low consumption of beta carotene is associated with high risk of developing cancer. Also smokers have low beta carotene levels in oral mucosa cells compared to nonsmokers. In several laboratory
and animal models, including hamster, these agents had strongly stopped oral cancer [29].
Studies in rats have shown that vitamin E could slow cancer cells progression. So this theory that vitamin E may have useful effects on head and neck cancer cells, has been already presented [29]. Also using vitamin E combined with mineral selenium and beta carotene decrease pain in radiotherapy of oral and intestinal membrane [29].
However there are studies that reject Vitamin E role in preventing cancer. In 1994, the National Cancer Institute reported that using antioxidant vitamins has not decreased lung cancers and other cancers in 29000 smokers. In 2002, Canadian researchers after 8 years study reported that antioxidants were not only unable to prevent secondary intestinal cancer, but secondary cancer rate was also reported two times more than that in the control group.
Likewise, the research done by the American Medical Association showed no significant decrease in mortality rate due to intestinal cancers in individuals using antioxidant substances. They also found that although a combination of vitamin E and beta carotene may have a protective effect on radiotherapy side effects, high dose of these antioxidants may interfere with the ability of radiotherapy to kill the cancer cells [30].
Our study results show that cigarette and alcohol significantly decreased saliva total protein and peroxides enzyme, but Vitamin E and C had protective effect and prevented the overwhelming changes happening in saliva composition. Fujinam et al. demonstrated that cigarette smoke causes total saliva protein and peroxides enzyme in rats that is similar to the above study results [9].
The saliva is a liquid that is exposed to gases like cigarette smoke. Free radicals, active oxygen, nitrogen species, carcinogens, and different toxic substances in cigarette smoke, expose oral epithelial cells to direct and constant attack and their gradual accumulation may end in malignant transformation [9, 14]. In a published study in 1997 by Dayan et al., it was demonstrated that saliva significantly inhibits initiating and progressing of oral cancer in an animal model induced by carcinogen factor, 4-nitroquinoline1-oxide [31]. Nishioka et al. provided further support for saliva anti-carcinogenic potentials against oral cancer [32].
The enzymes play the main role in oral defense mechanism, especially against the free radicals due to cigarette smoke. Saliva antioxidant system, especially peroxides has attracted a lot of attention. Studies have shown that oral peroxides enzyme inhibits initiating and progressing of oral cancer [33, 34]. According to recent observations, cigarette smoke causes 60% decrease in oral peroxides activity and since the saliva could not replace the damaged enzymes, loss of peroxides activity is irreversible.
Heavy smokers that smoke more than 20 cigarettes a day (since they lacked a recovery before their next smoking), show a clear decrease in peroxides enzyme activity. So their saliva is not protected by peroxides against cigarette smoke and other free radicals that penetrate into the oral cavity through foods, drinks, and inhaling substances [35, 31]. Studies have shown that passive smoking (indirect cigarette breathing) is more dangerous than smoking cigarette (mainstream smoking). Thus passive smoking biological effects could be worse than smoking the cigarette itself [36].
Like smokers, the rats exposed to cigarette smoke had a lower weight compared to the control group rats which is similar to Fujinam et al. study results. It is clear that loss of weight is due to exposure to cigarette smoke. Loss of weight in smokers is attributed to possible change in celery restorations or an increase in metabolic rate [37, 38].
In rats exposed to cigarette smoke or alcohol, vasodilatation, inflammatory cells, and hyperemia in parotid gland was observed. Argacha et al. and Fujinam et al. showed that nicotine dilates parotid gland peripheral vessels [9, 39]. Also Maier et al. showed that nicotine causes severe morphologic and functional changes in salivary glands [40].
In this study, some of the parotid glands exposed to cigarette smoke and alcohol showed intercalated duct portions which are consistent with Fujingham et al. study findings [9]. In Maier et al. study (1988), the effect of long-term alcohol and cigarette use on salivary glands function and morphology was considered. This study showed that chronic use of alcohol and cigarette smoking cause morphological and functional changes in salivary glands.
They noticed the fat accumulation in acini cells with decrease in weight and parotid gland protein contents. Nevertheless, clinical enlargement of parotid gland may be seen in chronic alcoholics [17]. Right now, tobacco is not classified as a carcinogen for salivary glands and little evidence exists about the connection between cigarette smoke and salivary glands tumor. In this study, there was no evidence supporting that salivary glands inflammation due to smoking could cause cancer.
5. Conclusion
The results of this study showed that vitamin E and C have both useful properties regarding cigarette and alcohol harmful effects and vitamin E effect was stronger than vitamin C.
Ethical Considerations
Compliance with ethical guidelines
The study was designed as a experimental study with the ethical code of IR.kmu.ac.ir 910212. The study was approved by the Institutional Human Research and Ethics Committee of Kerman University of Medical Sciences, Kerman, Iran.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no conflict of interest.
References
Studies in rats have shown that vitamin E could slow cancer cells progression. So this theory that vitamin E may have useful effects on head and neck cancer cells, has been already presented [29]. Also using vitamin E combined with mineral selenium and beta carotene decrease pain in radiotherapy of oral and intestinal membrane [29].
However there are studies that reject Vitamin E role in preventing cancer. In 1994, the National Cancer Institute reported that using antioxidant vitamins has not decreased lung cancers and other cancers in 29000 smokers. In 2002, Canadian researchers after 8 years study reported that antioxidants were not only unable to prevent secondary intestinal cancer, but secondary cancer rate was also reported two times more than that in the control group.
Likewise, the research done by the American Medical Association showed no significant decrease in mortality rate due to intestinal cancers in individuals using antioxidant substances. They also found that although a combination of vitamin E and beta carotene may have a protective effect on radiotherapy side effects, high dose of these antioxidants may interfere with the ability of radiotherapy to kill the cancer cells [30].
Our study results show that cigarette and alcohol significantly decreased saliva total protein and peroxides enzyme, but Vitamin E and C had protective effect and prevented the overwhelming changes happening in saliva composition. Fujinam et al. demonstrated that cigarette smoke causes total saliva protein and peroxides enzyme in rats that is similar to the above study results [9].
The saliva is a liquid that is exposed to gases like cigarette smoke. Free radicals, active oxygen, nitrogen species, carcinogens, and different toxic substances in cigarette smoke, expose oral epithelial cells to direct and constant attack and their gradual accumulation may end in malignant transformation [9, 14]. In a published study in 1997 by Dayan et al., it was demonstrated that saliva significantly inhibits initiating and progressing of oral cancer in an animal model induced by carcinogen factor, 4-nitroquinoline1-oxide [31]. Nishioka et al. provided further support for saliva anti-carcinogenic potentials against oral cancer [32].
The enzymes play the main role in oral defense mechanism, especially against the free radicals due to cigarette smoke. Saliva antioxidant system, especially peroxides has attracted a lot of attention. Studies have shown that oral peroxides enzyme inhibits initiating and progressing of oral cancer [33, 34]. According to recent observations, cigarette smoke causes 60% decrease in oral peroxides activity and since the saliva could not replace the damaged enzymes, loss of peroxides activity is irreversible.
Heavy smokers that smoke more than 20 cigarettes a day (since they lacked a recovery before their next smoking), show a clear decrease in peroxides enzyme activity. So their saliva is not protected by peroxides against cigarette smoke and other free radicals that penetrate into the oral cavity through foods, drinks, and inhaling substances [35, 31]. Studies have shown that passive smoking (indirect cigarette breathing) is more dangerous than smoking cigarette (mainstream smoking). Thus passive smoking biological effects could be worse than smoking the cigarette itself [36].
Like smokers, the rats exposed to cigarette smoke had a lower weight compared to the control group rats which is similar to Fujinam et al. study results. It is clear that loss of weight is due to exposure to cigarette smoke. Loss of weight in smokers is attributed to possible change in celery restorations or an increase in metabolic rate [37, 38].
In rats exposed to cigarette smoke or alcohol, vasodilatation, inflammatory cells, and hyperemia in parotid gland was observed. Argacha et al. and Fujinam et al. showed that nicotine dilates parotid gland peripheral vessels [9, 39]. Also Maier et al. showed that nicotine causes severe morphologic and functional changes in salivary glands [40].
In this study, some of the parotid glands exposed to cigarette smoke and alcohol showed intercalated duct portions which are consistent with Fujingham et al. study findings [9]. In Maier et al. study (1988), the effect of long-term alcohol and cigarette use on salivary glands function and morphology was considered. This study showed that chronic use of alcohol and cigarette smoking cause morphological and functional changes in salivary glands.
They noticed the fat accumulation in acini cells with decrease in weight and parotid gland protein contents. Nevertheless, clinical enlargement of parotid gland may be seen in chronic alcoholics [17]. Right now, tobacco is not classified as a carcinogen for salivary glands and little evidence exists about the connection between cigarette smoke and salivary glands tumor. In this study, there was no evidence supporting that salivary glands inflammation due to smoking could cause cancer.
5. Conclusion
The results of this study showed that vitamin E and C have both useful properties regarding cigarette and alcohol harmful effects and vitamin E effect was stronger than vitamin C.
Ethical Considerations
Compliance with ethical guidelines
The study was designed as a experimental study with the ethical code of IR.kmu.ac.ir 910212. The study was approved by the Institutional Human Research and Ethics Committee of Kerman University of Medical Sciences, Kerman, Iran.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no conflict of interest.
References
- Homann N, Tillonen J, Rintamäki H, Salaspuro M, Lindqvist C, Meurman JH. Poor dental status increases acetaldehyde production from ethanol in saliva: A possible link to increased oral cancer risk among heavy drinkers. Oral Oncology. 2001; 37(2):153-8. [DOI:10.1016/S1368-8375(00)00076-2]
- La Vecchia C, Tavani A, Franceschi S, Levi F, Corrao G, Negri E. Epidemiology and prevention of oral cancer. Oral Oncology. 1997; 33(5):302-12. [DOI:10.1016/S1368-8375(97)00029-8]
- Brugere J, Guenel P, Leclerc A, Rodriquez J. Differential effects of tobacco and alcohol in cancer of the larynx, pharynx and mouth. Cancer. 1986; 57(2):391-5. [DOI:10.1002/1097-0142(19860115)57:23.0.CO;2-Q]
- Franceschi S, Talamini R, Barra S, Baron AE, Negri E, Bidoli E, et al. Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx and esophagus in Northern Italy. Cancer Research . 1990; 50(20):6502-7. [PMID]
- Graham S, Dayal H, Rohrer T, Swanson M, Sultz H, Shedd D, et al. Dentition, diet, tobacco, and alcohol in the epidemiology of oral cancer. Journal of the National Cancer Institute. 1977; 59(6):1611-8. [DOI:10.1093/jnci/59.6.1611] [PMID]
- Reznick AZ, Klein I, Eiserich JP, Cross CE, Nagler RM. Inhibition of oral peroxidase activity by cigarette smoke: In vivo and in vitro studies. Free Radical Biology and Medicine. 2003; 34(3):377-84. [DOI:10.1016/S0891-5849(02)01297-2]
- Squier CA, Mantz MJ, Wertz PW. Effect of menthol on the penetration of tobacco carcinogens and nicotine across porcine oral mucosa ex vivo. Nicotine & Tobacco Research. 2010; 12(7):763-7. [DOI:10.1093/ntr/ntq084] [PMID]
- Nagler RM, Barak M, Peled M, Ben-Aryeh H, Filatov M, Laufer D. Early diagnosis and treatment monitoring roles of tumor markers Cyfra 21-1 and TPS in oral squamous cell carcinoma. Cancer. 1999; 85(5):1018-25. [DOI:10.1002/(SICI)1097-0142(19990301)85:53.0.CO;2-R]
- Fujinami Y, Fukui T, Nakano K, Ara T, Fujigaki Y, Imamura Y, et al. The effects of cigarette exposure on rat salivary proteins and salivary glands. Oral Diseases. 2009; 15(7):466-71. [DOI:10.1111/j.1601-0825.2009.01572.x] [PMID]
- Nagler R, Lischinsky S, Diamond E, Drigues N, Klein Y, Reznick AZ. Effect of cigarette smoke on salivary proteins and enzyme activities. Archives of Biochemistry and Biophysics. 2000; 379(2):229-36. [DOI:10.1006/abbi.2000.1877] [PMID]
- Densen PM, Davidow B, Bass HE, Jones EW. A chemical test for smoking exposure. Archives of Environmental Health: An International Journal. 1967; 14(6):865-74. [DOI:10.1080/00039896.1967.10664853] [PMID]
- Nagler R, Lischinsky S, Diamond E, Drigues N, Klein Y, Reznick AZ. New insights into salivary lactate dehydrogenase of human subjects. Journal of Laboratory and Clinical Medicine. 2001; 137(5):363-9. [DOI:10.1067/mlc.2001.114710] [PMID]
- Moore S, Calder KAC, Miller NJ, Rice-Evans CA. Antioxidant activity of saliva and periodontal disease. Free Radical Research. 1994; 21(6):417-25. [DOI:10.3109/10715769409056594] [PMID]
- Zappacosta B, Persichilli S, De Sole P, Mordente A, Giardina B. Effect of smoking one cigarette on antioxidant metabolites in the saliva of healthy smokers. Archives of Oral Biology. 1999; 44(6):485-8. [DOI:10.1016/S0003-9969(99)00025-4]
- Hoffman WP, Ness DK, Van Lier RB. Analysis of rodent growth data in toxicology studies. Toxicological Sciences. 2002; 66(2):313–9. [DOI:10.1093/toxsci/66.2.313] [PMID]
- Nogueira-Filho Gda R, Rosa BT, Cesar-Neto JB, Tunes RS, Tunes Uda R. Low- and high-yield cigarette smoke inhalation potentiates bone loss during ligature-induced periodontitis. Journal of Periodontology. 2007; 78(4):730-5. [DOI:10.1902/jop.2007.060323] [PMID]
- Maier H, Born IA, Veith S, Adler D, Seitz HK. The effect of chronic ethanol consumption on salivary gland morphology and function in the rat. Alcoholism: Clinical and Experimental Research. 1986; 10(4):425-7. [DOI:10.1111/j.1530-0277.1986.tb05117.x] [PMID]
- Maier H, Born IA, Mall G. Effect of chronic ethanol and nicotine consumption on the function and morphology of the salivary glands. Wiener Klinische Wochenschrift. 1988; 11:140-50. [PMID]
- Moriguchi S, Kobayashi N, Kishino Y. High dietary intakes of vitamin E and cellular immune functions in rats. The Journal of Nutrition. 1990; 120(9):1096-102. [DOI:10.1093/jn/120.9.1096] [PMID]
- Djurašević SF, Djordjević J, Drenca T, Jasnić N, Cvijić G. Influence of vitamin C supplementation on the oxidative status of rat liver. Archives of Biological Sciences. 2008; 60(2):169-73. [DOI:10.2298/ABS0802169D]
- Wang PL, Shirasu S, Shinohara M. Salivary amylase activity of rats fed a low calcium diet. The Japanese Journal of Pharmacology. 1997; 78(3):279-83. [DOI:10.1254/jjp.78.279]
- Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and maxillofacial pathology. St Louis: Saunders; 2009.
- Truelson J. Oral cancer: Diagnosis, management, and rehabilitation. Plastic and Reconstructive Surgery. 2008; 121(2):673-4. [DOI:10.1097/01.prs.0000307725.05814.ae]
- Rothman k, Keller A. The effect of joint exposure to alcohol and tobacco on risk of cancer of the mouth and pharynx. Journal of Chronic Diseases. 1972; 25(12):711-6. [DOI:10.1016/0021-9681(72)90006-9]
- Schottenfeild D. Alcohol as a co-factor in the etiology of cancer. Cancer. 1979; 43(S5):1962-6. [DOI: 10.1002/ 1097-0142(197905)43:5+<1962::aid-cncr2820430703>3.0.co;2-p]
- Blot WJ, Maclaughlin JK, Winn DM. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Research. 1988; 48(11):3282-7. [PMID]
- Bross IDJ, Cooms J. Early onset of oral cancer among woman who smoke and drink. Oncology. 1976; 33(3):136-9. [DOI:10.1159/000225127] [PMID]
- Bundgard T, Bentzen SM , Wildt J . The prognostic effect of tobacco and alcohol consumption in intra oral squamous cell carcinoma. European Journal of Cancer Part B: Oral Oncology. 1994; 30(5):323-8. [DOI:10.1016/0964-1955(94)90033-7]
- Garewal HS. Beta-carotene and vitamin E in oral cancer prevention. Journal of Cellular Biochemistry. 1993; 53(S17F):262-9. [DOI:10.1002/jcb.240531039] [PMID]
- Lippman SM, Goodman PJ, Klein EA, Parnes HL, Thompson IM Jr, Kristal AR, et al. Designing the selenium and vitamin E cancer prevention trial. Journal of the National Cancer Institute. 2005; 97(2):94-102. [DOI:10.1093/jnci/dji009] [PMID]
- Dayan D, Hirshberg A, Kaplan I, Rotem N, Bodner L. Experimental tongue cancer in desalivated rats. Oral Oncology. 1997; 33(2):105-9. [DOI:10.1016/S0964-1955(96)00048-6]
- Nishioka H, Nishi K, Kyokane K. Human saliva inactivates mutagenicity of carcinogens. Mutation Research/Environmental Mutagenesis and Related Subjects. 1981; 85(5):323-33. [DOI:10.1016/0165-1161(81)90223-5]
- Sariri R, VarastehA, Erfani A, Rezaei A, Heidari Z. Inhibition of salivary peroxidase by cigarette smoke. Health. 2010; 2(4):347-51. [DOI:10.4236/health.2010.24052]
- Knak R, Rutsatz K, Gocke R. Salivary investigations in oral lichen planus. Journal of Dental Research. 1998; 77:27-38.
- van der Vliet A, Nguyen MN, Shigenada MK, Eiserich JP, Marelich GP, Cross CE. Myeloperoxidase and protein oxidation in cystic fibrosis. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2000; 279(3):L537-46. [DOI:10.1152/ajplung.2000.279.3.L537] [PMID]
- Byrd JC. Environmental tobacco smoke. Medical Clinics of North America. 1992; 76(2):377–98. [DOI:10.1016/S0025-7125(16)30358-3]
- Wack JT, Rodin J. Smoking and its effects on body weight and the systems of caloric regulation. The American Journal of Clinical Nutrition. 1982; 35(2):366-80. [DOI:10.1093/ajcn/35.2.366] [PMID]
- Gordon T, Kannel WB, Dawber TR, McGee D. Changes associated with quitting cigarette smoking: the Framingham Study. American Heart Journal. 1975; 90(3):322–8. [DOI:10.1016/0002-8703(75)90320-8]
- Argacha JF, Adamopoulos D, Gujic M, Fontaine D, Amyai N, Berkenboom G, et al. Acute effects of passive smoking on peripheral vascular function. Hypertension. 2008; 51(6):1506–11. [DOI:10.1161/HYPERTENSIONAHA.107.104059] [PMID]
- Sadetzki S, Oberman B, Mandelzweig L, Chetrit A, Ben-Tal T, Jarus-Hakak A, et al. Smoking and risk of parotid gland tumors: A nationwide case-control study. Cancer. 2008; 112(9):1974-82. [DOI:10.1002/cncr.23393] [PMID]
Type of Study: Original article |
Subject:
Surgery
Received: 2017/08/20 | Accepted: 2017/11/10 | Published: 2018/01/1
Received: 2017/08/20 | Accepted: 2017/11/10 | Published: 2018/01/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |